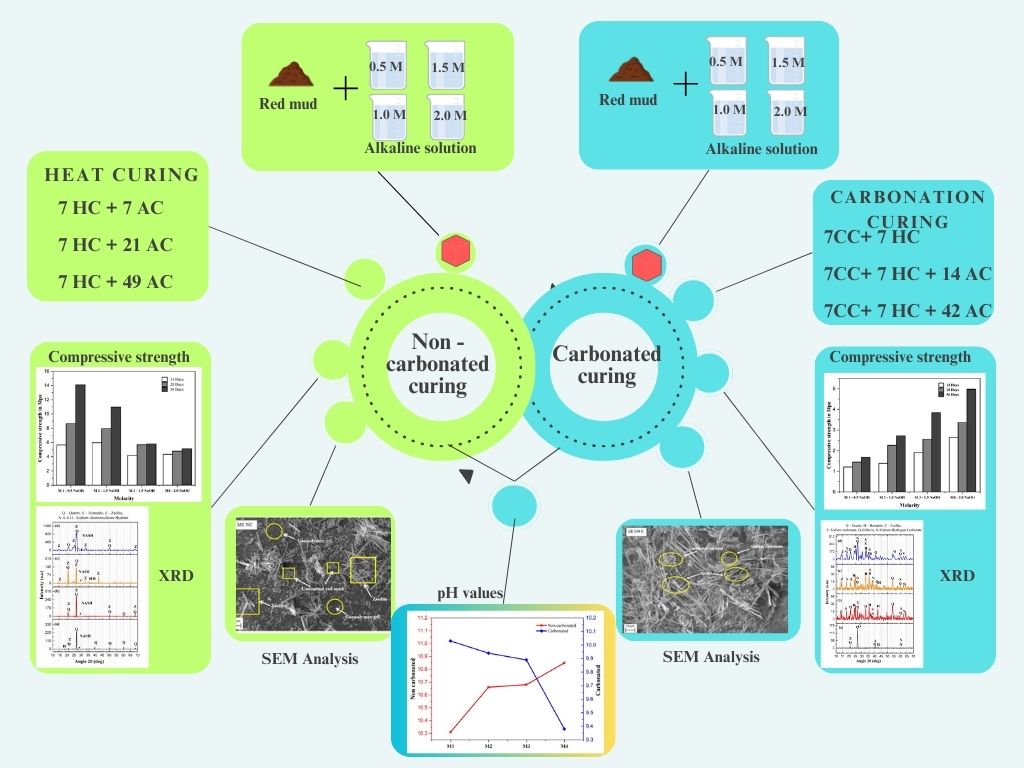
This research explores using low molarity sodium hydroxide (NaOH) in alkali activated red mud based geopolymer mortars to enhance sustainability. Carbonation curing was employed to capture CO2 and promote mineral carbonation. The study assessed how NaOH molarity (0.5M to 2M) affects physical, mechanical, microstructural properties and statistical analysis through compressive strength, pH, crystalline characterization, microstructural analysis before and after carbonation and ANOVA analysis. Non-carbonated samples showed highest compressive strength at 0.5M NaOH, while carbonated samples peaked at 2M. Red mud's natural alkalinity enhanced strength at low NaOH concentrations in non-carbonated conditions. Carbonated samples showed greater strength at 2M due to higher Na⁺ availability. XRD analysis identified geopolymer reaction products and unreacted phases in non-carbonated samples, while carbonated ones showed sodium carbonate. SEM showed dense N-A-S-H gel at 0.5M in non-carbonated samples and zeolite needle formations and sodium carbonate crystals in carbonated samples at 2M. pH increased with higher NaOH concentrations in non-carbonated samples and decreased after carbonation. ANOVA revealed curing period affects compressive strength of red mud geopolymer mortar, with M1-M3 gaining strength while M4 stabilized early. Results indicate red mud's alkalinity enables effective geopolymerization at lower NaOH concentrations, while higher NaOH concentration enhances CO2 sequestration through carbonation curing, highlighting potential for sustainable applications.
Total file downloads: 10