- Cite article
- Download PDF
- Share article
- 15 Downloads
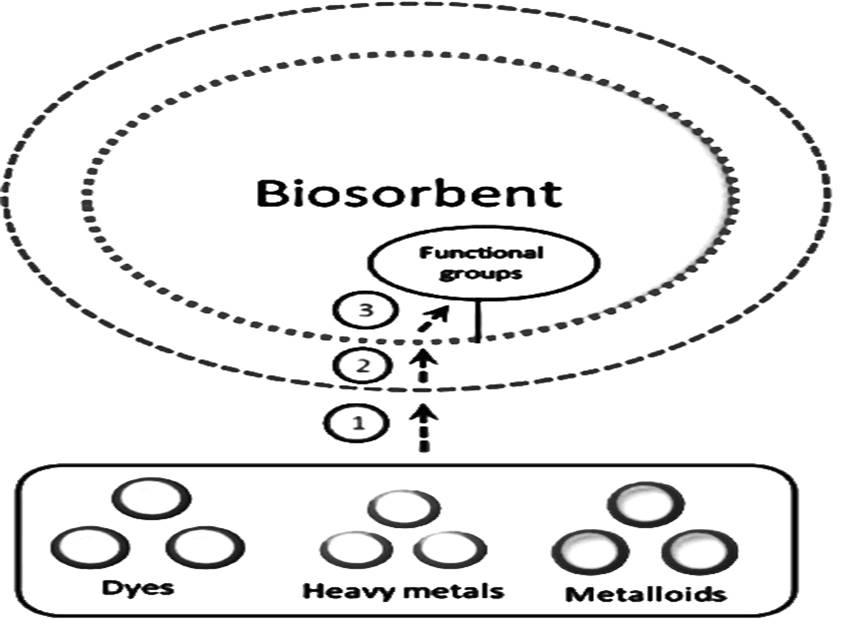
The biosorption of azo dyes from the synthetic aqueous solutions waswere investigated using the Prosopis Juliflora Root Powder (PJRP). The experiments involved varying the initial dye concentrations (10 – 50 mg/L), the reaction time of 90 minutes, pH levels rangingdye solution’s pH level varied from 2.0 to 7.0, and sorbent dosages from 1 to 5 g/20mL. The original dye solution's temperature and ionic strength, among other variables influencing the absorption process, were also investigated. Adsorption isotherms for Temkin, Langmuir, and to replicate the adsorption data, Freundlich was employed. R2 > 0.95 for CR, CV, and MB, respectively, show that they fit relatively well with the initial state data of the biosorption technique progression. The adsorption kinetics were confirmed using first and second-order pseudo models. The assessment of thermodynamic constants, such as ΔG°, ΔH°, and ΔS°, was carried out. It was discovered that the two dyes had endothermic dye adsorption processes. At an optimal pH, the highest adsorption capacity Qo for MB was 92.4 mg/g, while for CV, it was 88.9 mg/g, and for CR, it was 82.26 mg/g.