- Cite article
- Download PDF
- Share article
- 36 Downloads
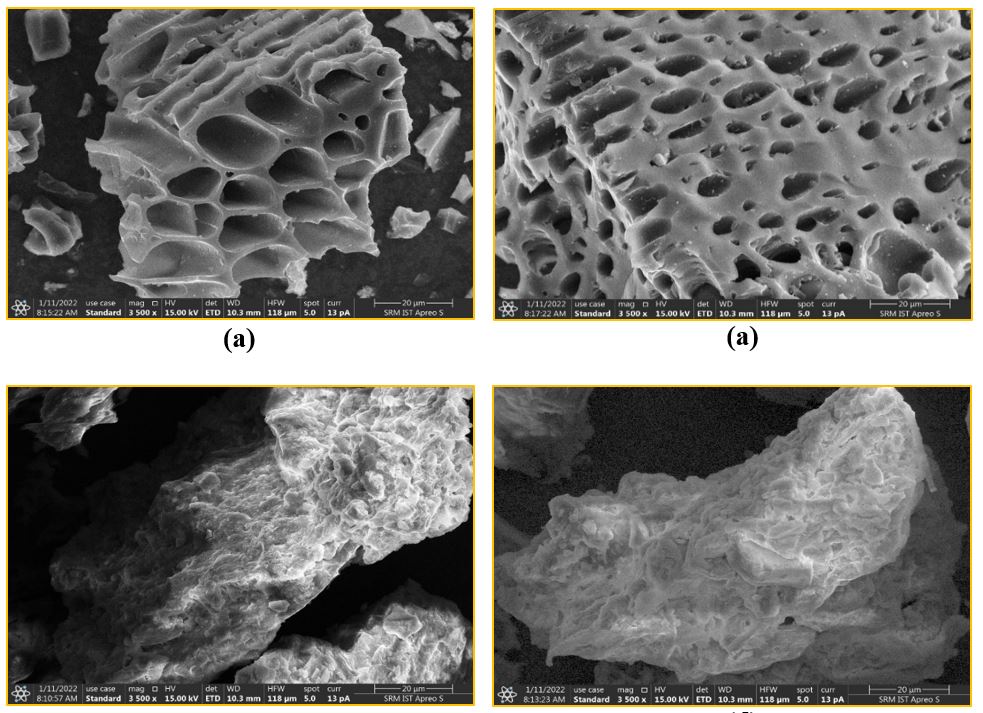
The study aimed to analyze the adsorption behaviour of an activated rice husk biochar adsorbent in batch mode to expunge azo dye concentrations depletion from liquid solvents. The biochar adsorbent was formulated using different chemical synthesis processes, and its surface region was assessed using Nitrogen retention. The existence of the particular azo dyes was verified through SEM and EDX examinations. Additionally, XRD analysis was conducted to examine the intensity and crystalline structure of the activated biochar adsorbent. A batch mode study was conducted to ascertain the ideal adsorption parameters, including pH, contact time, biochar dose, and azo dye concentration. The adsorption kinetic studies supported the notion of a favourable adsorption interaction occurring through the mutual influence of the adsorbed matter and the adsorbing substrate. Most isotherm studies were well-fitted with regression values of isotherm models, indicating an adsorption process with non-uniform monolayer formation. Thermodynamic investigations validated that the binding forces between the adsorptive and the adsorbing phase are endothermic, and a significant amount of spent adsorbent was successfully recovered using a strong hydrochloric acid desorption process.