- Cite article
- Download PDF
- Share article
- 9 Downloads
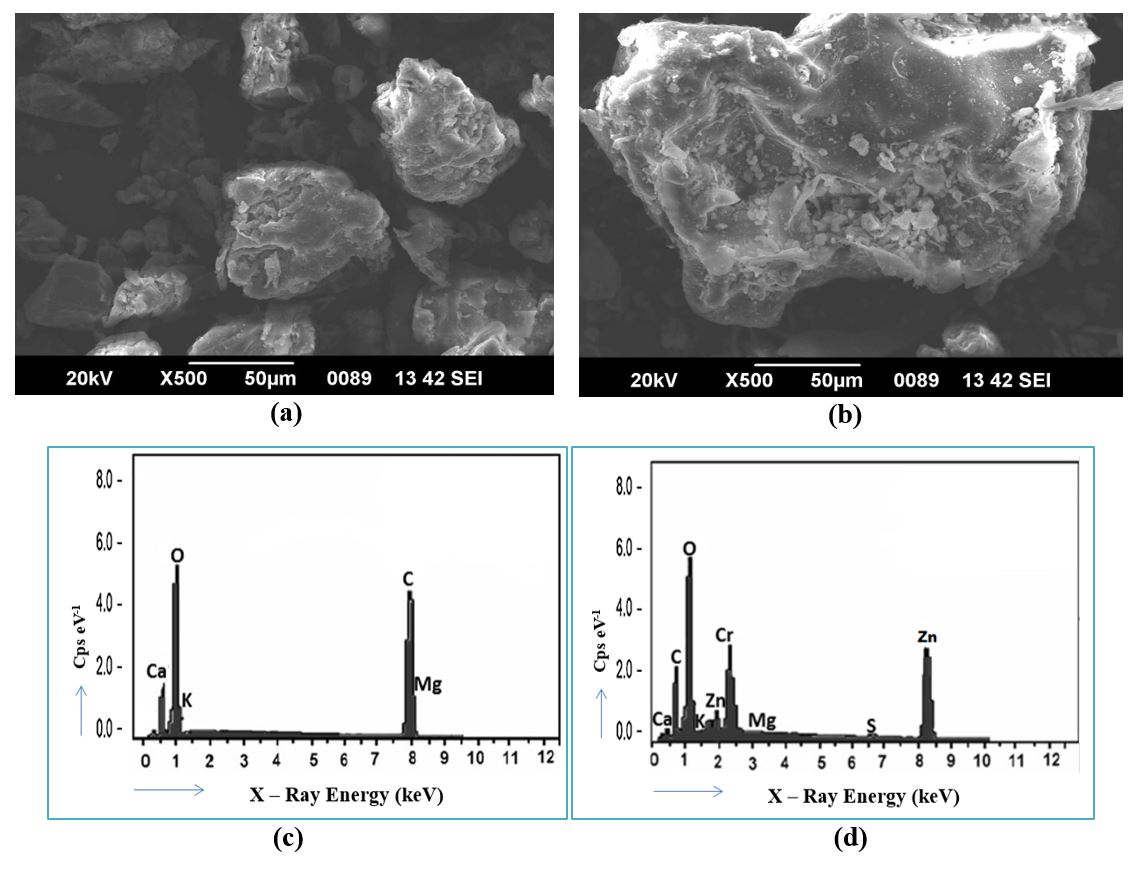
In this study, a batch adsorption process was conducted utilizing Ascophyllum Nodosum biosorbent as an organic adsorbent used for the elimination of heavy metal ions (specifically chromium, nickel, and zinc) from wastewater. To examine the surface morphology and functional groups, the biosorbent underwent analysis using FTIR, SEM & EDX technologies. To understand the nature of the adsorption process, several isothermal studies were conducted. These studies aimed to determine whether the adsorption process was homogeneous or heterogeneous in nature. Furthermore, the physical or chemical nature of the metal ion adsorption on the adsorbent was investigated through kinetic studies. Thermodynamic investigations were also carried out to assess whether the adsorption of metal ions was exothermic or endothermic, as well as to conclude the spontaneity of the process. Notably, the findings indicated that the inclusion of 0.3N HCl resulted in the highest rate of desorption, indicating its effectiveness in releasing the adsorbed metal ions. The AN – Brown Algae adsorbs targeted metal ions (Cr(VI), Ni(II), Zn(II)) at percentage of 93.55%, 87.56% and 83.27% respectively from the synthetic solution under the optimum conditions in batch study.