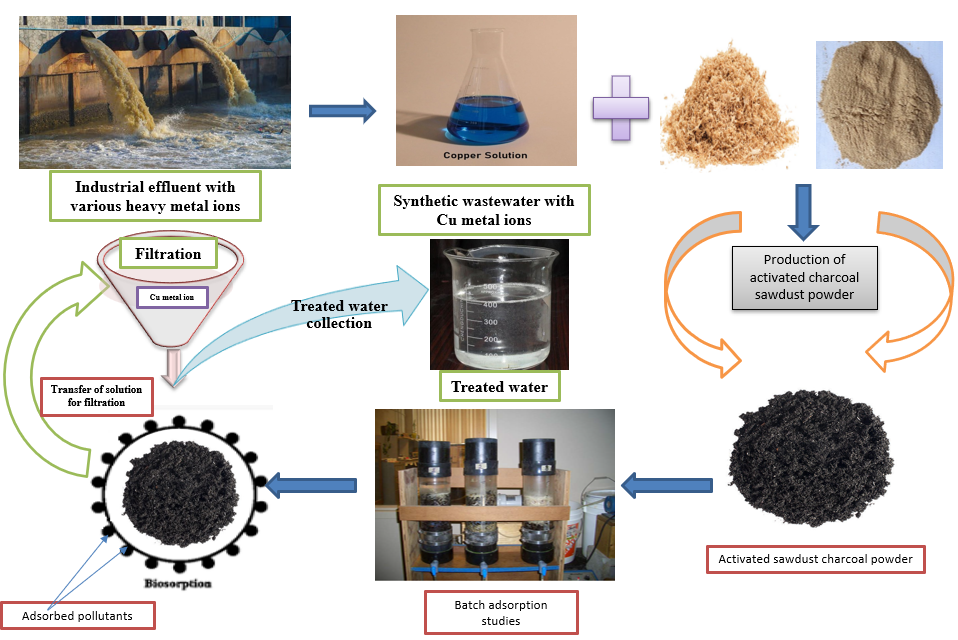
The batch adsorption technique investigated the efficiency of sawdust adsorbent for removing copper ions from synthetic solutions. A chemical synthesis process prepared the activated sawdust powder, and its surface area and pore volume were obtained by BET isotherm analysis. The ability of copper ion uptake by activated sawdust powder was examined under the characterization study of SEM, EDX & FTIR studies. Various isotherm studies checked the process of adsorption, and the kinetic studies confirm the nature of the adsorption process with sawdust adsorbent. Thermodynamic studies were used to analyze the endothermic nature of the adsorption process, and 0.3 N of H2SO4 acid desorbs 93.27% of copper ions from the spent adsorbent. The experimental study confirms the better adsorption ability of sawdust powder in the activated charcoal form to remove the heavy metal pollution in aqueous solutions.
Total file downloads: 10