- Cite article
- Download PDF
- Share article
- 52 Downloads
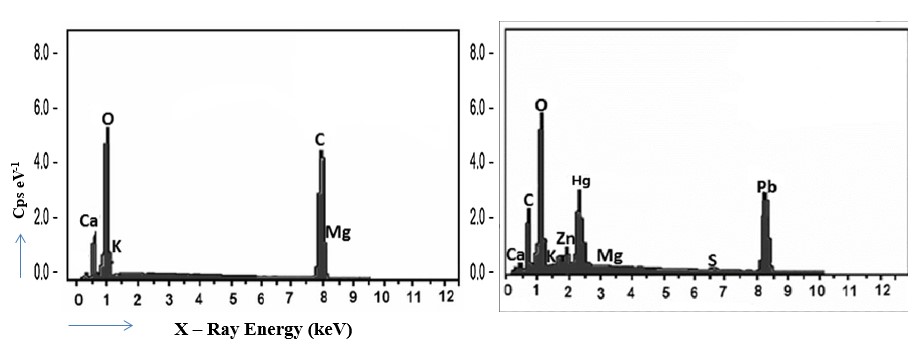
The water hyacinth biosorbent has undergone thorough characterization and assessment for its capacity to remove heavy metals, specifically Pb(II) and Hg(II), from aqueous solutions. Notably, the biosorbent demonstrated an impressive maximum adsorption capacity for Pb and Hg, reaching values of 158.7 mg/g and 123.5 mg/g, respectively. Indeed, these adsorption capacities align with or even surpass those documented in the existing literature for various biosorbents derived from similar agro-industrial waste materials. Several methods were used to characterize the water hyacinth biosorbent's structure and morphology, including pH-Zero Point Change, scanning electron microscopy/energy-dispersive X-ray spectroscopy (SEM/EDS), and infrared spectrometry with Fourier transform (FT-IR). The water hyacinth biosorbent was found to have broken exteriors with mixed particles that involved useful adsorption clusters such as OH, C=O, and C-O-C. The most effective adsorption of Pb(II) and Hg(II) takes place at a pH of 5.0, using a hyacinth dose of 2 g/L. The chemisorption process is the primary cause of the fast growth rate of the adsorption process, which is governed by pseudo-second-order kinetics. Using a 0.1M HNO3 eluent, both biosorbents were effectively regenerated, enabling effective reuse for up to five cycles. The thermodynamic studies confirm the endothermic nature of the adsorption process.