- Cite article
- Download PDF
- Share article
- 2 Downloads
ABSTRACT
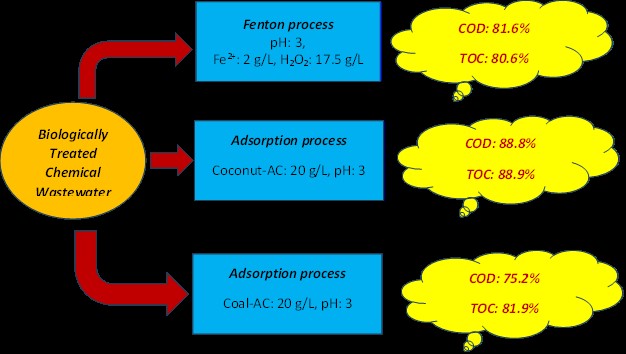
In this study, the advanced treatment of biologically treated chemical industrial wastewater from organic peroxide production was investigated using Fenton and adsorption processes. In the Fenton oxidation process, wastewater treatment was performed at different Fe²⁺ and H₂O₂ concentrations, pH values, and oxidation times to determine the optimal treatment conditions and treatment kinetics. In the adsorption process, wastewater treatment was conducted using coconut-based activated carbon (Coconut-AC) and coal-based activated carbon (Coal-AC) at different pH values, activated carbon doses, and adsorption times to determine the best treatment conditions, adsorption kinetics, and isotherms. In Fenton oxidation, COD (4680 mg/L), TOC (1022 mg/L), and organic peroxide removal were 81.6%, 80.6%, and 77.3%, with 1 h oxidation at pH 3, 2 g/L Fe2+, and 17.5 g/L H2O2, respectively. In the adsorption process, better wastewater removal was achieved with Coconut-AC than with Coal-AC. At pH 3, with a 20 g/L activated carbon dose, 88.8% COD, 88.9% TOC, and 86.4% organic peroxide removal were obtained with Coconut-AC after 24 h of adsorption, while 75.2% COD, 81.9% TOC, and 54.5% organic peroxide removal were observed with Coal-AC. Although the SO42- concentration in the wastewater increased with Fenton oxidation, 30.8% SO42- removal was observed with Coconut-AC and 10.6% with Coal-AC. The cost was 15.77 $/m3 in Fenton oxidation under optimal conditions, compared to 15.87 $/m3 in the adsorption processes with Coconut-AC and Coal-AC. As a result, the adsorption process with Coconut-AC achieved higher wastewater treatment efficiency than Fenton oxidation, while the cost per m³ of wastewater remained nearly the same. Moreover, SO₄ removal was effectively achieved in the adsorption process
Keywords: Adsorption, COD removal, cost analysis, Fenton oxidation, organic matter removal, TOC removal, organic peroxide, wastewater treatment