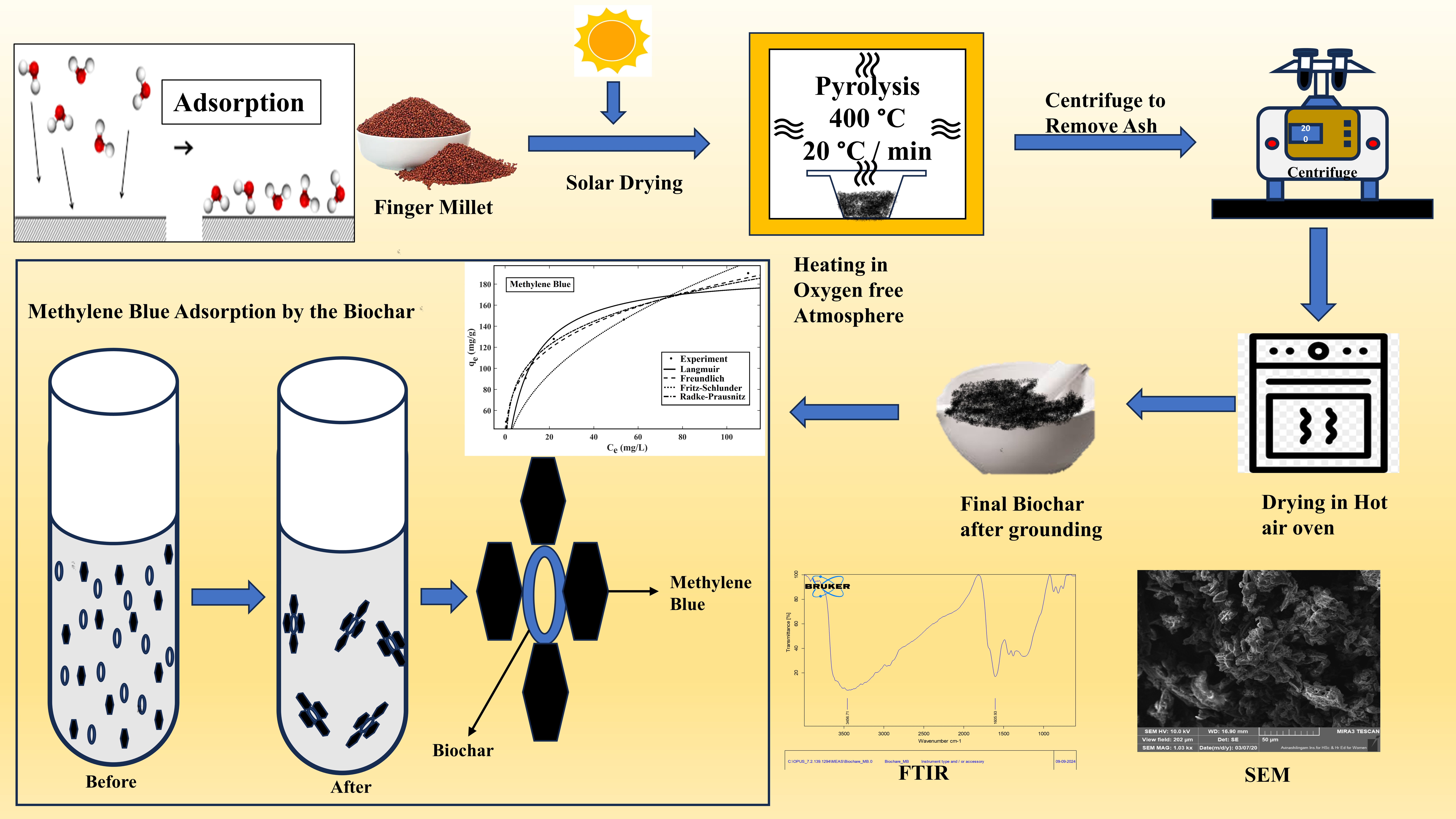
Globally, synthetic dyes contribute to significant environmental contamination due to their toxicity, durability, and resistance to conventional treatment processes. Therefore, there is a need to adopt best practices for remediation. Our study examines the potential of biochar produced from Finger Millet Waste Biomass (FMWB) as an effective and environmentally sustainable adsorbent material for the uptake of Methylene Blue (MB) from aqueous solutions. The characterisation of the biochar's physicochemical properties- specifically, surface morphology, porosity, and functional groups- is conducted using Fourier Transform Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD), Scanning Electron Microscopy coupled with Energy Dispersive X-ray Spectroscopy (SEM-EDX), Particle Size Analysis (PSA), and zeta potential. The adsorption processes are systematically optimised, taking into account various factors such as pH, sorbent dose, initial dye concentration, contact time, and temperature. The adsorption efficiency for MB correlates with the initial increase of biochar dose, reaching a saturation plateau at 1 g/L, with 100% removal observed at an optimal pH of 7–8, and the adsorbent exhibited a maximum adsorption capacity of 191.8 mg/g confirming its potential as a sustainable adsorbent for dye-laden wastewater. As the feed dye concentration increases, adsorption efficiency decreases, suggesting monolayer adsorption dominates at lower concentrations. Isotherm analysis suggests that the Freundlich model best described the process (with 𝑅2=0.9877), indicating heterogeneous multilayer adsorption. Kinetic modeling confirms chemisorption, with a pseudosecond-order mechanism being predominant. Thermodynamic analysis reveals the exothermic and spontaneous nature of the adsorption process. The findings indicate that biochar derived from finger millet possesses a substantial potential for application as an adsorbent in wastewater treatment processes. This is attributed to its notable adsorption efficiency, economic viability, and sustainability attributes.
Total file downloads: 11