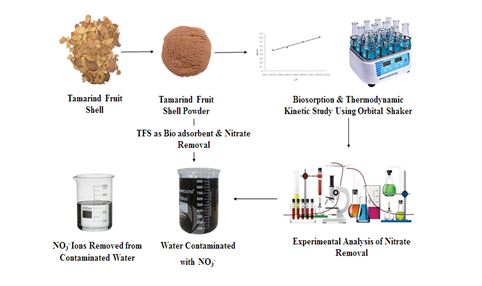
The majority of nitrate (NO3‾) pollutants come from a different type of industries. Even if present in low amounts, these nitrates are harmful to humans and aquatic systems. This research investigated the utilisation of Tamarind Fruit shell (TFS) as biosorbent in the removal of Nitrate from aqueous solution. TFS shell was modified by soaking it with distilled water. Many remediation strategies are available, but they are both costly and ineffectual. However, it has been discovered via significant research that waste materials such as tamarind bark, eggshells, and agricultural wastes are well suited to removing nitrate from waste water using the biosorption process (Panida, et.al., 2010). Biosorption is a nitrate removal process that is both environment friendly and cost-effective. The equilibrium distribution of NO3‾ onto the surface of the TFS can be best explained by Freundlich isotherm. Based upon the reaction of the thermodynamic study the TFS- NO3‾ interaction is an endothermic (Amardeep, et.al., 2013). A substantial chance in the NO3‾ - TFS interaction is expected because of the significant gain in the translational entropy by the displaced synchronised water molecules. Than the Chemiluminescence Analysing techniques Spectroscopic studies shows the biosorption capacity of TFS shows a little variation between 1.25 mg/g for 1 mm size and 2.14 mg/g for 0.13 mm size.
Total file downloads: 4