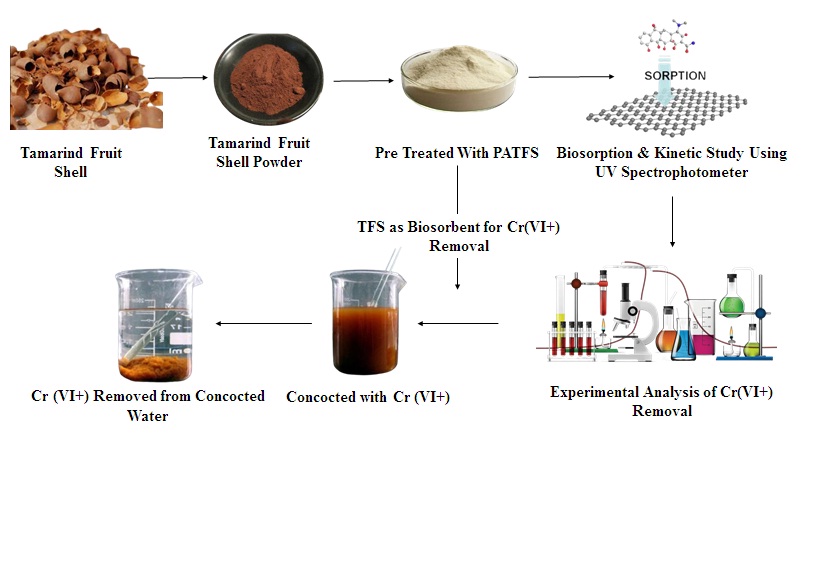
The locally available tamarind fruit shells (TFS) pre-treated with 1N H3PO4 was used to remove the hexavalent chromium (Cr6+) from aqueous phase. The investigation was done by using continuously mixed batch reactor (CMBR) under room temperature. Few preliminary investigations was undertaken to optimize the system parameters of the Cr6+ PATFS system. The batch studies cover the important experiments such as biosorption kinetics, biosorption equilibrium isotherm, effect of pH, effect of ionic strength, and effects of the presence of other anions in the system on biosorption of Cr6+. From preliminary investigation, the PATFS revealed a highest efficacy (99.1%) in removing Cr6+ from aqueous phase, apart from other pre-treated TFS. It was revealed that the density and mechanical properties of the treated and untreated composites increased with the decrease in the particle size; whereas, the water absorption percentage and void content decreased for the same. (a) Effect of heavy metal concentration on the sorption capacity. (b) Effect of solution pH on the sorption capacity. (c) Effect of sorption temperature on the sorption capacity. (d) Effect of sorption time on the sorption capacity. At a concentration of 50 mg/l of Cr6+, more than 95% was appeared to be removed by 12 g/l of 0.13 mm (geometric mean size) of PATFS. The biosorption kinetics of Cr6+ was modeled by using integrated rate expression and second order rate constants were determined for different Cr6+concentrations. Uptake of Cr6+on PATFS was well described by Freundlich isotherm for different biosorbent sizes. UV-VIS spectrophotometer was used to measure the concentration of Hexavalent chromium in water. The desorption study was also carried out and it’s significant by 0.5H2SO4.
Total file downloads: 22