- Cite article
- Download PDF
- Share article
- 20 Downloads
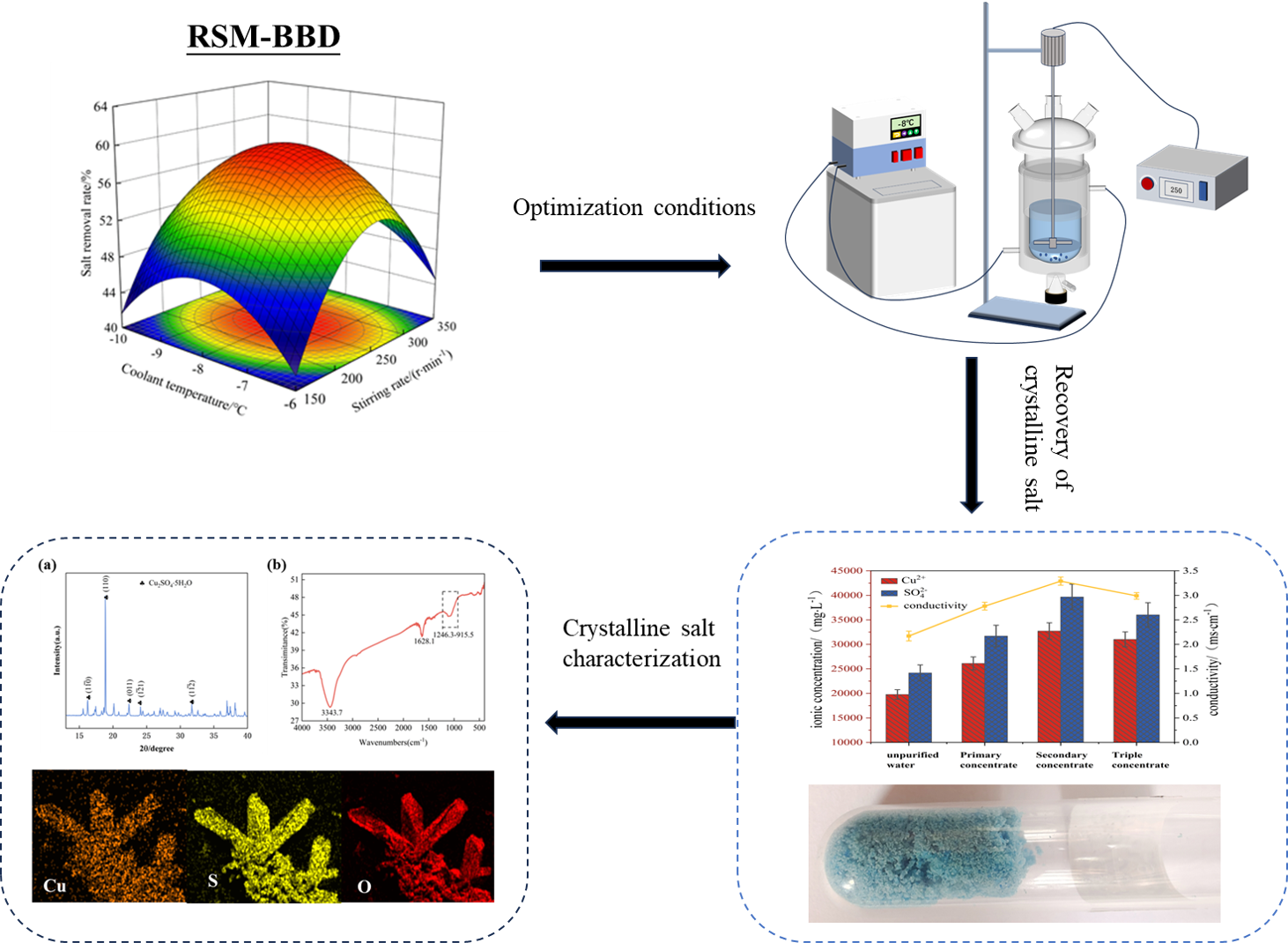
For the copper-containing electroplating wastewater generated by a semiconductor company, the recovery of copper salts by progressive freeze crystallization and its process parameters were experimentally optimized and studied. Based on the results of single-factor experiments, according to the principles of Box-Behnken Design experimental design for response surface methodology to investigate the effects of coolant temperature, agitation rate, and freezing time on salt removal and optimize process parameters. The optimization results of the response surface method showed that the optimal conditions were when the coolant temperature was -8°C, the stirring rate was 264 r×min-1, and the freezing time was 78 min. Three validation experiments were conducted under optimum conditions, and salt removal was obtained as 60.13 ± 4.10%, which is more in line with the predicted values. Treatment of copper-containing electroplating wastewater using tertiary freezing under response surface optimized experimental conditions. At this time, the total ice rate of the wastewater was about 65.00%, and the conductivity was also concentrated from 2.17 mS×cm-1 of the raw water to 2.99 mS×cm-1 with a total concentration ratio of 1.38. In addition, a higher concentration of concentrated liquid was obtained in the third freezing, while a crystalline salt precipitated from the bottom of the reactor. The precipitate was analyzed mainly as copper sulfate pentahydrate using XRD, XRF, EDS, and other characterization techniques. Theoretical calculations show that pre-cooling of raw wastewater reduces energy consumption by up to 20.40%.