- Cite article
- Download PDF
- Share article
- 28 Downloads
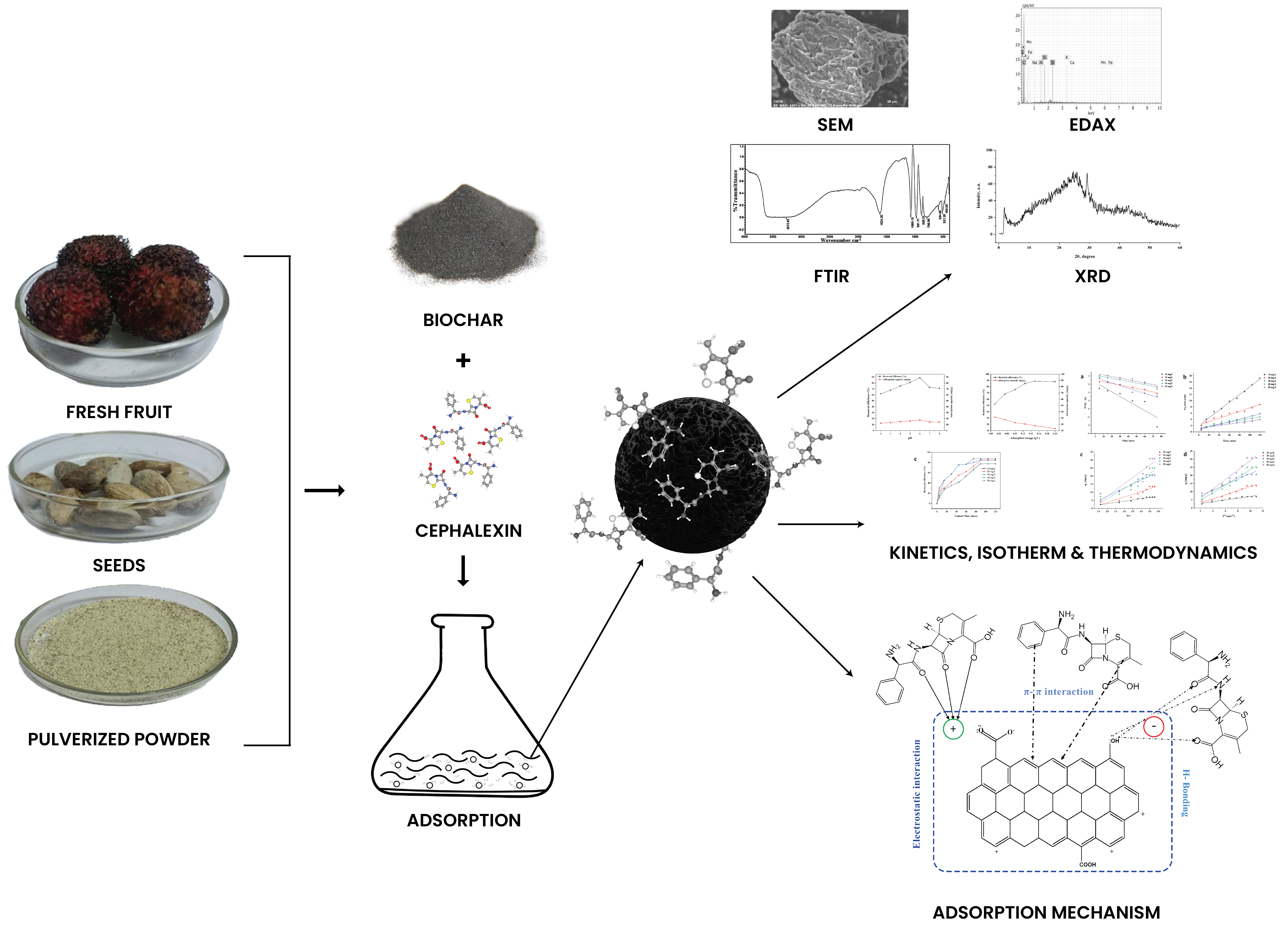
The improper disposal of drugs into bodies of water has become a severe environmental and health issue. Biochar synthesized from different agro residues could be a potential material for the adsorption of pollutants in aqueous environments. Nephelium lappaceum seeds were pyrolyzed at 600 °C in a pyrolysis reactor incorporating a slow pyrolysis process for carbonization. Fourier-Transform Infrared (FTIR), Scanning Electron Microscope (SEM), and X-ray diffraction (XRD) were used to characterize the biochar before and after adsorption of the pharmaceutical pollutant cephalexin. The effects of several factors, including initial cephalexin concentration, time of contact, dosage of adsorbent, and pH, were considered for the sorption. The findings showed that the Freundlich model offered the greatest fit for adsorption. The better-fitted kinetic model was the pseudo-second-order kinetic. Based on Langmuir isotherm model, the maximum adsorption capacity of cephalexin was obtained as 63.69 mg/g. The adsorption process involves hydrogen bonding, surface complexation, electrostatic and π−π EDA interactions. The current study offers a feasible and optimistic method for using agricultural waste and an alternative adsorbent substance for recovering highly concentrated cephalexin from water-based solutions.