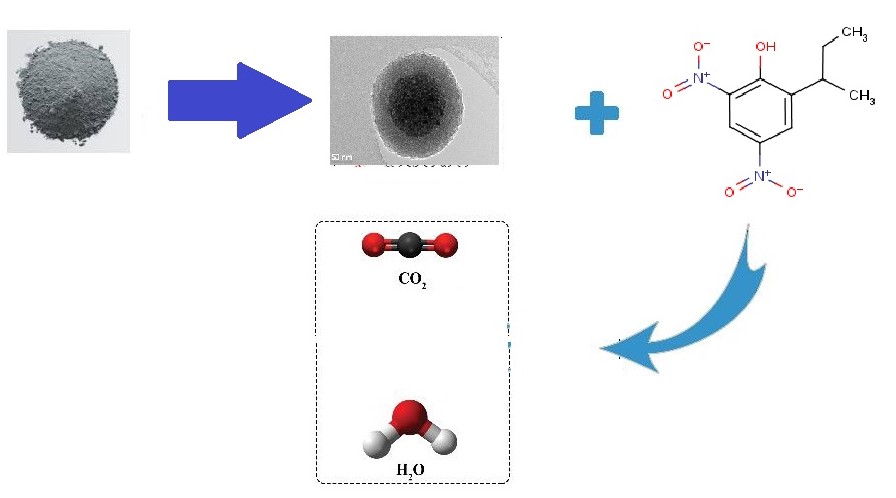
Dinitrobutylphenol is a toxic and resistant pollutant that pollutes the environment by producing toxins and chemical wastewater. Present paper aims to study the efficiency of the magnetic particles of walnut hard shell ash as a cheap and bi-compatible adsorbent for the removable of DNBP from aqueous environments. This study was conducted at laboratory scale and studied the most important parameters including contact time, pH, adsorbent dose, concentration of the pollutant and temperature on the efficiency of the process. Walnut hard shell ash was magnetized and its properties were studied using SEM, FTIR, XRD, and BET techniques. The fitness of the data was studied using Langmuir and Freundlich isotherms. Results showed that at pH=7, temperature 25°C , 700 mg of adsorbent, within 50 min, the removal efficiency of DNBP with the initial concentration of 20 mg reached 93%. The isotherm studies showed that this process with fitness coefficient of 99% conforms to Langmuir equation and it has higher conformity to maximum adsorption of 142.88 mg/g. Magnetic particles of walnut hard shell ashes as a natural bio-friendly and cheap adsorbent is able to efficiently remove DNBP as a toxic and persistent material from aqueous environments and industrial wastewaters.
Total file downloads: 3