- Cite article
- Download PDF
- Share article
- 5 Downloads
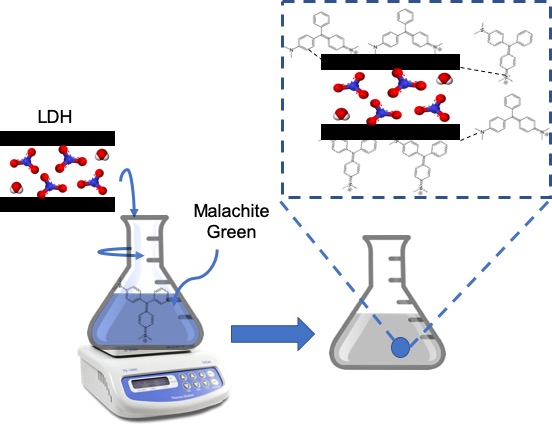
Layered double hydroxides (LDHs) of MgM3+ (M3+=Al and Cr) were synthesized by coprecipitation method to form Mg/Al and Mg/Cr LDHs. The materials were applied as adsorbents of malachite green in aqueous solution. The physical properties of Mg/Al and Mg/Cr were analyzed using XRD, FTIR, BET and TGDTA characterizations. The XRD pattern shows the characteristic of LDHs which has diffraction at 11.470 (003) and at 34.690 (012) for Mg/Al and 12.450 (003) and at 380 (012) for Mg/Cr. The interlayer spaces of Mg/Al and Mg/Cr LDHs were 7.71 Å and 7.62 Å, respectively. The surface area of Mg/Al was higher than Mg/Cr. The FTIR spectra confirm that the intense peak at 1385 cm-1 denotes vibration of nitrate bond and M-O band in under 1000 cm-1. Thus the Mg/Al and Mg/Cr LDHs were applied as adsorbents to remove malachite green in aqueous solution. The results of malachite green adsorption showed that malachite green was adsorbed onto Mg/Al and Mg/Cr followed pseudo second order and Langmuir adsorption parameter. The adsorption capacity of malachite green for Mg/Al and Mg/Cr was 44.444 mg/g and 33.784 mg/g, respectively. The thermodynamic study showed that the adsorption process was spontaneous, endothermic and favored in high temperature. The regeneration process showed that Mg/Al and Mg/Cr LDHs has high stability structure toward reusability of adsorbent until three cycles adsorption process.