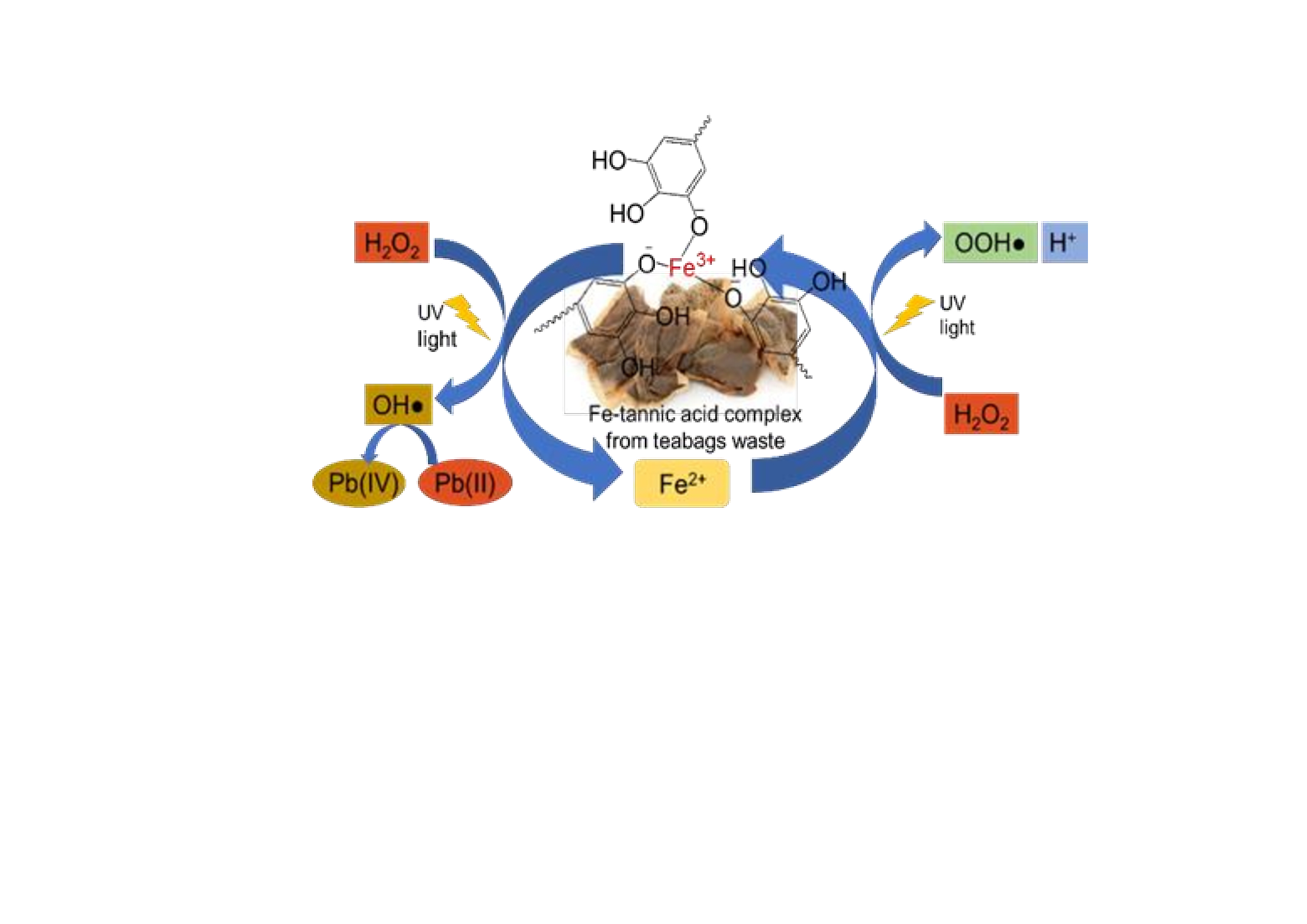
IIntroducing tannic acid from tea leaves waste as a complexing agent of Fe(III) into photo-Fenton process to prevent Fe(OH)3 precipitation at pH 7, and further to increase photo-Fenton activity in the near neutral pH for Pb(II) photo-oxidation is systematically addressed. The photo-Fenton process for Pb(II) photo-oxidation involving Fe2+ and H2O2 solutions as well as UV light was conducted by batch technique, both in the absence and present of tannic acid. Furthermore, the optimization of tannic acid concentration, solution pH, and reaction time were also conducted. The research results assign that the addition of tannic acid in the photo-Fenton process for photo-oxidation of Pb(II) is able to considerably enhance the effectiveness at pH 6-8 (near neutral pH). The activity of the tannic acid from tea leaves waste is comparable to the that of the commercial tannic acid. The best condition of Pb(II) photo-oxidation having 10 mg.L-1 in 50 mL of the solution can be reached by using Fe2+ as high 10 mmol.L-1, H2O2 100 mmol.L-1, with the addition of tannic acid as much as 30 mg.L-1 at pH 7 and 60 min of the time that gave about 85% of the photo-oxidation effectiveness. It is also indicated that the Pb(II) photo-oxidation has resulted in the harmless PbO2 solid . Hence, it is obviously assigned that the priceless tea waste could significantly contribute in the improvement of the photo-Fenton performance to prevent environmental pollution.
Total file downloads: 25