- Cite article
- Download PDF
- Share article
- 11 Downloads
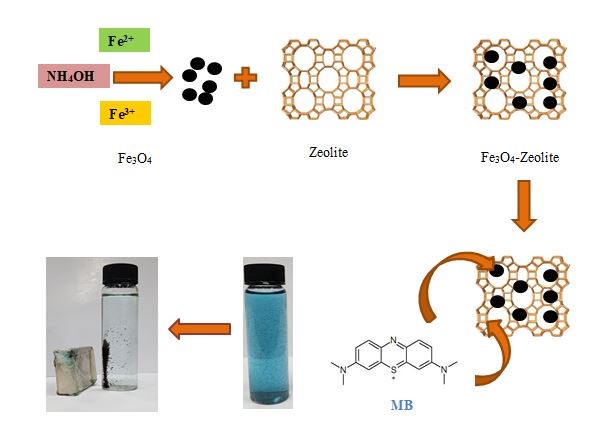
In this paper, the low-cost and practical adsorption for removing methylene blue (MB) dye has been developed by using recoverable natural zeolite that was magnetized with Fe3O4. The magnetization was conducted by co-precipitation technique. The adsorbents obtained from the magnetization were characterized by XRD, FTIR, surface area analyzer and turbidity meter machines. The MB adsorption on the recoverable adsorbent was performed by batch experiment. The effect of Fe3O4 fraction on adsorbent characters, recoverability, and adsorption ability was evaluated. The adsorption kinetic and isotherm were also determined. The research results attributed that recoverable zeolite/Fe3O4 adsorbent has been successfully produced. It was found that the increase of Fe3O4 fraction in the adsorbent, has improved the recoverability, but in the same time, it caused the adsorption decreased. The fraction of Fe3O4 as much 33.30%w displayed compromisingly good capacity and recoverability. The maximum MB dye adsorption was reached by a condition of 0.25 g L-1 of the adsorbent dose, pH 8, and in 60 mins of the contact time The adsorption kinetic well fitted with pseudo second-order with the adsorption rate of 0.0238 mg g-1 min-1. The adsorption strongly agreed with the Langmuir isotherm with adsorption capacity of 32.258 mg g-1 .