- Cite article
- Download PDF
- Share article
- 36 Downloads
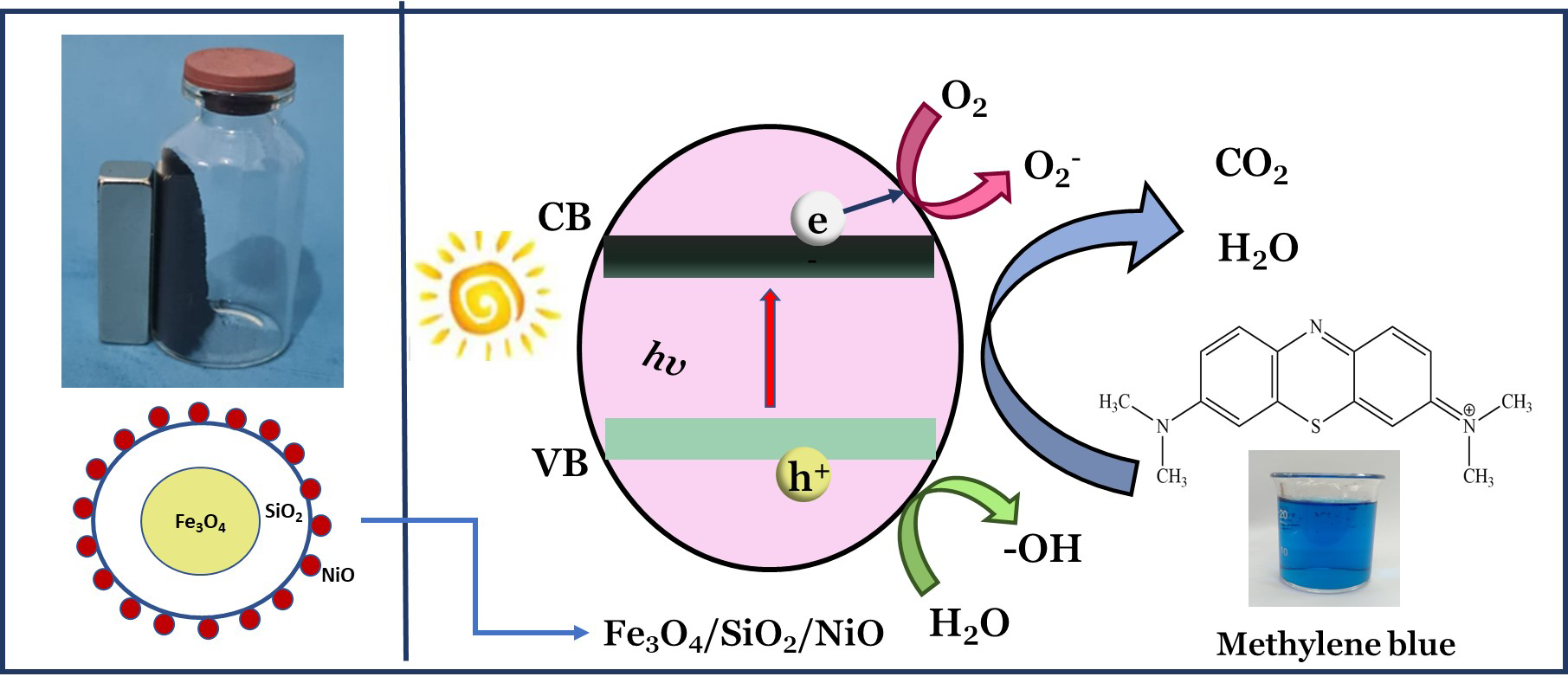
Photocatalytic degradation for wastewater treatment is a method that has recently attracted attention. In this research, a synthesized composite of Fe3O4/SiO2/NiO with magnetic properties was used for the photocatalytic degradation of methylene blue dye under UV light. Furthermore, the composites were characterized using XRD, FTIR, BET surface area, SEM-EDS, VSM, and UV-DRS. The results showed that the Fe3O4/SiO2/NiO composite is magnetic with a saturation magnetization of 53.84 emu/g. The Fe3O4/SiO2/NiO composite has a surface area of 128.8 m2/g, large than Fe3O4 and Fe3O/SiO2. The Fe3O4/SiO2/NiO composite has a band gap of 2.83 eV. The photocatalytic activity of Fe3O4/SiO2/NiO composite against the methylene blue dye exhibited high degradation efficiency reaching 98.51 %. The pseudo-first-order is appropriate to describe the kinetics model of photocatalytic degradation on methylene blue dye. The decrease in the degradation efficiency of the Fe3O4/SiO2/NiO composite after 5 times for the photocatalytic degradation of methylene blue dye from 98.02 % to 94.97 % indicates that the catalyst has high stability. Considering these results, the Fe3O4/SiO2/NiO composites could be used as a potential catalyst in industrial wastewater.