- Cite article
- Download PDF
- Share article
- 8 Downloads
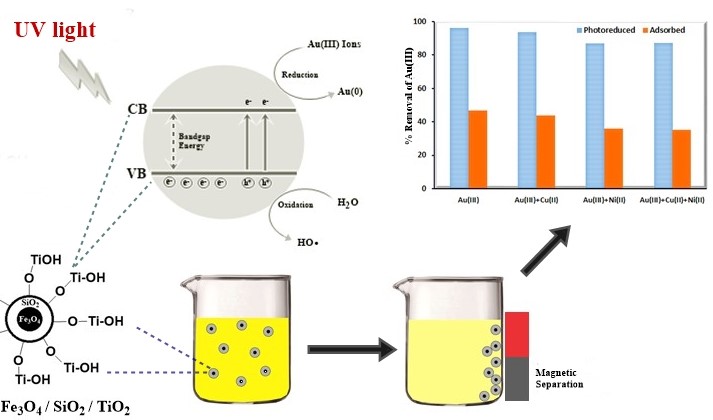
Metallurgy and recovery of gold in electronic waste sometimes involve the reduction of tetrachloroaurate ion (AuCl4-) to elemental gold form. Currently, for the reduction of tetrachloroaurate ion, people use reducing agents such as hydroquinone and sodium borohydride. Photocatalysts of Fe3O4/SiO2/TiO2 nanoparticles were prepared and tested for the reduction of tetrachloroaurate ion under UV light illumination. The magnetite (Fe3O4) nanoparticle was first prepared by coprecipitation and sonication, followed by SiO2 and TiO2 coatings via the sol-gel process and calcination. The products were confirmed by XRD and TEM. The photocatalytic reduction of tetrachloroaurate ion was performed in a closed reactor equipped with a UV light source. The results indicated that Fe3O4/SiO2/TiO2 nanoparticles were successfully prepared, which retained good magnetic and photocatalytic properties. The photocatalytic reaction is best performed at a pH of 5 under UV irradiation for 2 h, which is capable of reducing 96% of the tetrachloroaurate present in the mixture. The co-presence of Ni2+ and Cu2+ ions in the solution leads to a decrease in yield due to competitive reduction and adsorption. The photocatalyst is recoverable by the use of a magnetic bar and may find application for gold recovery and metallurgy.