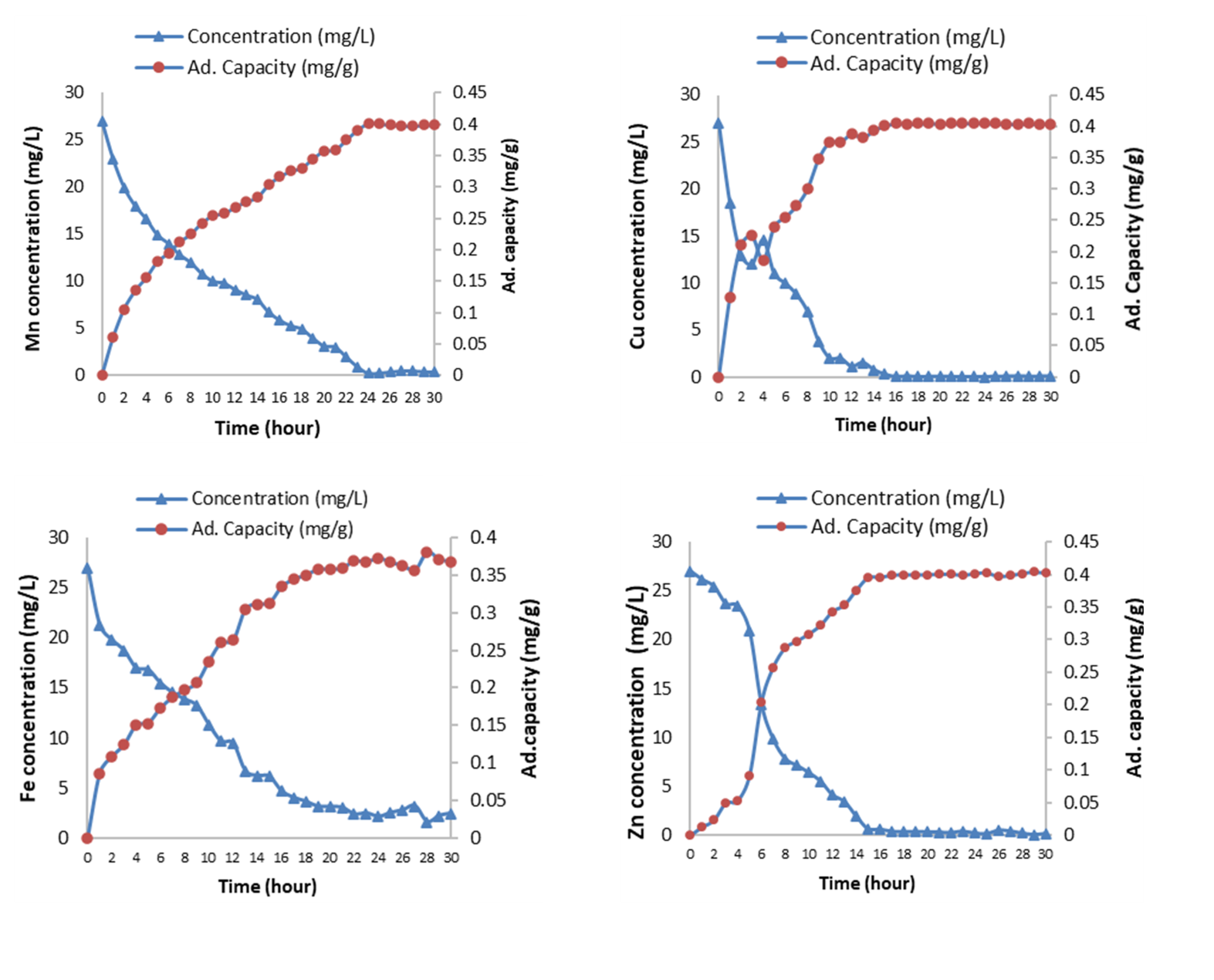
This study highlights the potential of steel slag, which is an industrial by-product of steel making industry as treatment media for metal-rich acid mine drainage (AMD). A series of batch adsorption studies has been done to demonstrate the effects of contact time, solution pH, initial concentration of metal, adsorbent dosage and size, and the effect of competing ions on the performance of steel slag. Results indicated that metal removal efficiencies were found to be >90% when pH of AMD has reached near-neutral state (6.8-7.5) that were mostly occurring within the first 14 hours of contact time. Optimum equilibrium time was found at 24 hours, i.e. 99-100% of metals were removed. An increased adsorption capacity with decreased removal efficiency was observed as initial metal concentration increased. In contrast, increasing adsorbent dosage leads to increased removal efficiency. Fe was not affected despite the presence of other metal ions (100% removal) compared to Mn (59.3% removal) in mixed AMD solution. Adsorption behavior of Fe, Cu, Zn and Mn fits appropriately with Langmuir isotherm model with adsorption capacity of 1.06, 1.03, 0.97 and 0.73 mg g-1, respectively. The adsorption kinetics followed the pseudo-second-order kinetics and is supported by intra-particle diffusion process. Therefore, steel slag can be potentially used as an effective media for passive AMD remediation.
Total file downloads: 4