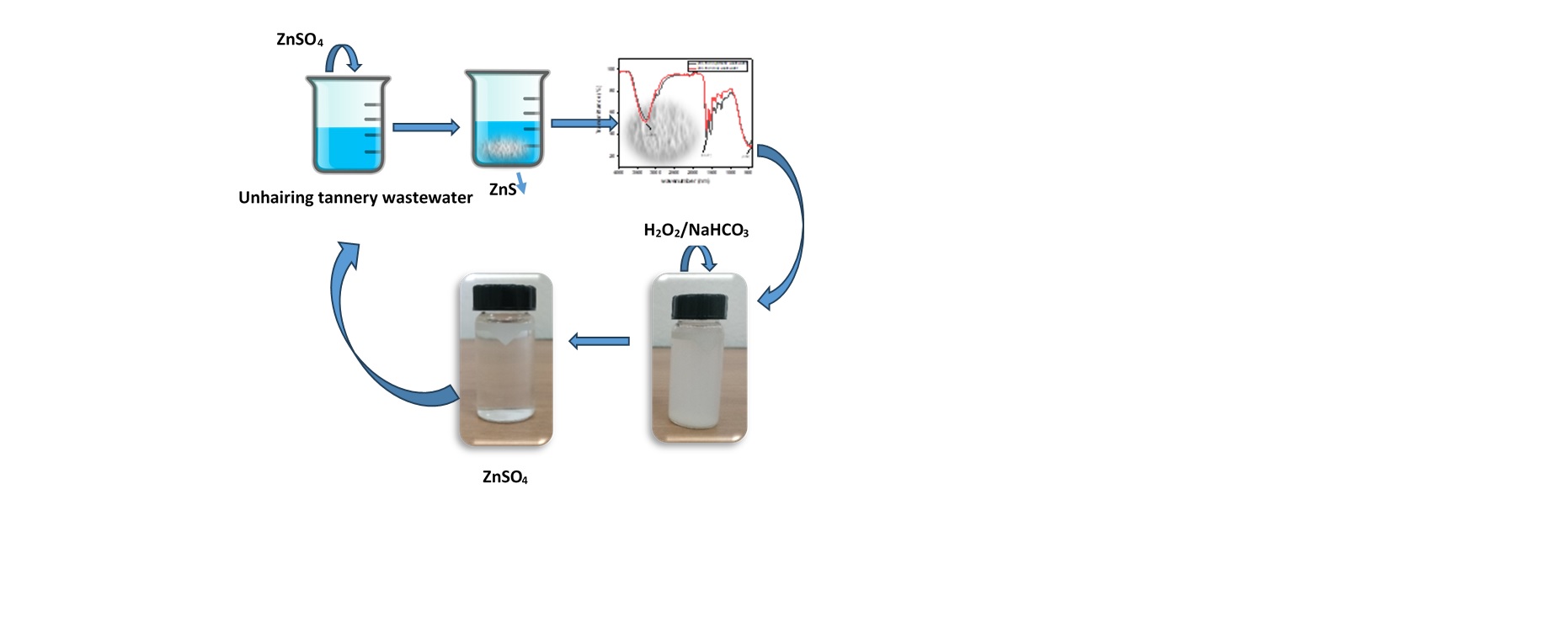
This study investigates the effectiveness of a combined precipitation and oxidation leaching process in reducing high concentrations of sulphides in tannery effluent from the unhairing bath. First, at precipitation process the influence of operational parameters, such as stirring speeds, pH levels, and different zinc salts, was evaluated on the precipitation settling rate of ZnS. Due to its high solubility product (pKs) and at optimal conditions, ZnS proved to be highly efficient, achieving up to a 95% reduction in sulphide ions concentration. Moreover, Analysis of the treated unhairing wastewater revealed significant improvements in water quality, including reductions in pH (from 12.4 to 8.11), and turbidity (88%). Additionally, chemical oxygen demand (COD) analysis indicated the preservation of valuable and recoverable nitrogenous organic compounds, such as proteins, peptides, and free amino acids, post-treatment. The FTIR spectra analysis confirmed the good quality of the obtained precipitate. On the other hand, when hydrogen peroxide (H₂O₂) was added to zinc sulfide (ZnS) in the presence of sodium bicarbonate (NaHCO₃) at ambient temperature, the leaching of ZnS occurred, promoting the oxidation of sulfide ions to sulfate (97%). This process effectively reduced metal sludge production. This clean method offers a promising zero-discharge strategy for sulfide mitigation in industrial wastewater treatment systems and could be broadly applied in environmental management practices
Total file downloads: 35