- Cite article
- Download PDF
- Share article
- 3 Downloads
The treatment of wastewater containing synthetic dyes represents an environmental challenge due to their complex molecular structures and high stability. This study investigates the heterogeneous photocatalytic degradation of Acid Orange 10 (AO10), an azo dye widely used in the textile industry, using titanium dioxide (TiO2, Degussa P25) under ultraviolet (UV) irradiation in both closed and semi-closed photoreactor configurations.
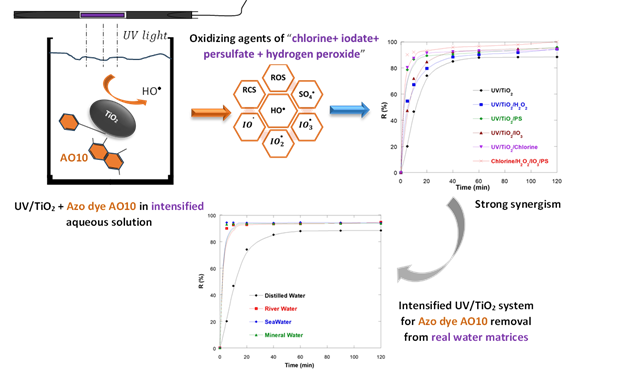
Parametric optimization revealed that optimal degradation was achieved at pH 6.5, 25 °C, and 0.1 g/L TiO2 for an initial dye concentration of 10 mg/L, reaching 88% removal efficiency after 120 min in the UV/TiO2 system.
Process intensification through the addition of oxidizing agents (H2O2, K2S2O8, Cl-, and IO3-) significantly enhanced degradation performance. Among all tested systems, UV/TiO₂/IO₃⁻ exhibited the most remarkable enhancement, achieving 97% removal in 120 min, while the combined UV/TiO2/IO3-/PS system achieved 97% removal in only 5 minutes, attributed to the synergistic generation of multiple reactive radicals (•OH, SO4•-, and IO3•).
Kinetic analysis confirmed that the degradation follows pseudo-first-order kinetics with excellent linearity (R² ≥ 0.99), with apparent rate constants increasing proportionally with oxidant addition.
Real matrix validation using river water, seawater, and mineral water confirmed the robustness of the process, maintaining >90% degradation efficiency despite the presence of interfering ions. These findings demonstrate that TiO2-based photocatalysis, intensified with oxidizing agents, represents an efficient and sustainable approach for treating dye-polluted effluents.