- Cite article
- Download PDF
- Share article
- 2 Downloads
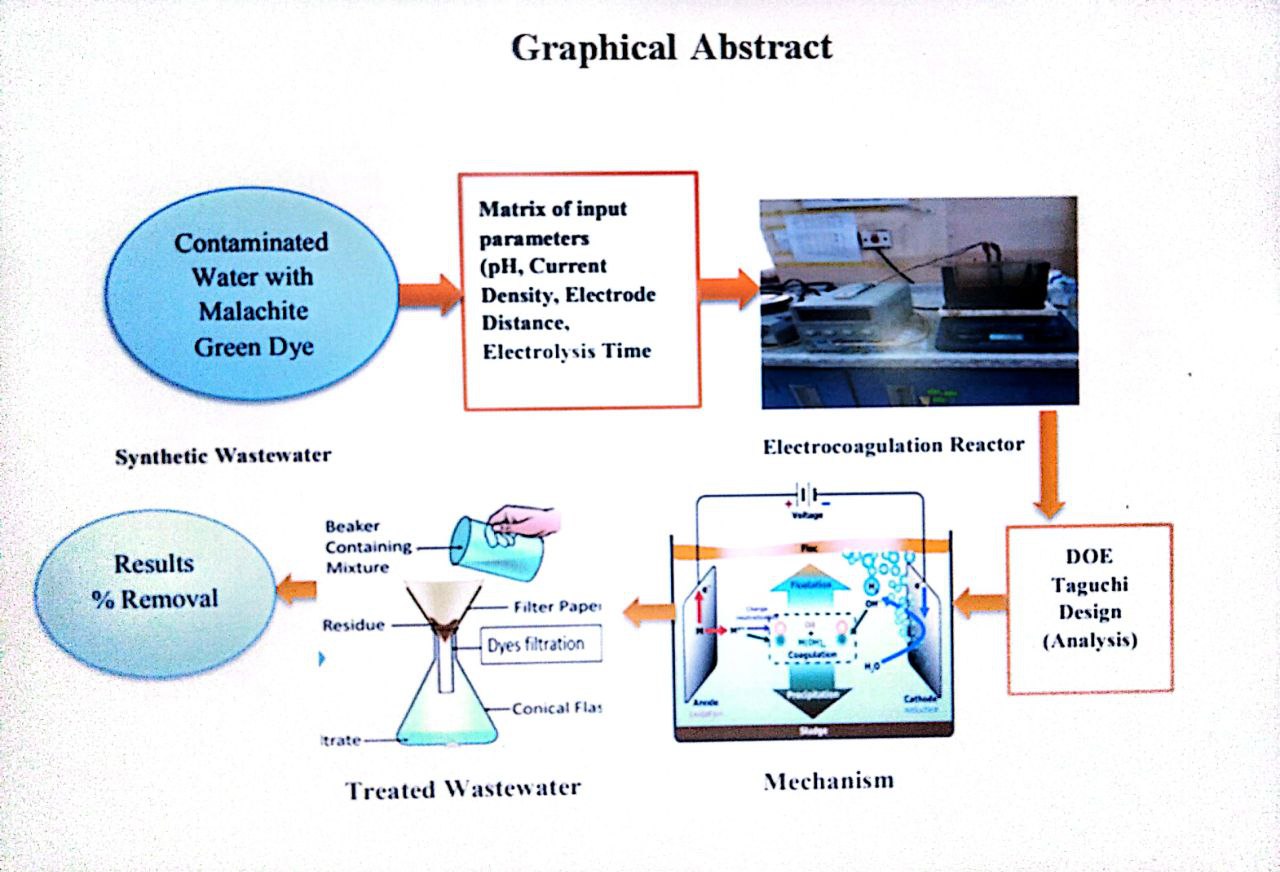
This study investigated the removal of Malachite Green (MG) from water through electrocoagulation (EC) using Fe–Fe, Al–Al, and Al–Fe electrode setups. The Taguchi method, employing an L25 (5^5) orthogonal array, focused on optimizing five key factors: current density (0.2–1.0 mA/cm²), pH (3–10), initial dye concentration (10–100 mg/L), distance between electrodes (5–25 mm), and duration (5–60 min). Signal-to-noise (S/N) ratio and ANOVA evaluations indicated that pH (10) with Fe-Fe electrodes and current density (0.8 mA/cm²) with Al-Al electrodes were the most significant variables. Increasing the initial dye concentration or the distance between electrodes raised the voltage and consistently increased the removal percentage (%R). Enhancing electrolyte concentration initially improved color removal %R to a certain extent, after which no further enhancements were observed. In terms of efficient MG removal from water, the Fe-Fe, Al-Al, and Fe-Al configurations demonstrated efficiencies ranging from 12.59% to 98.6%. The initial concentration had a substantial impact on results (with ANOVA contributions of 44.3% to 34.9%), closely followed by the duration of electrolysis. Optimal conditions yielded 88% to 100% removal with less than 3% deviation from predicted values. The maximum conditions for color removal varied based on electrode material: pH (10, 8, and 10), current density (1.0, 0.8, and 0.8 mA/cm²), contact time (20, 60,and 60 min), distance between electrodes (20, 15,and 15 mm), and initial concentration (100 mg/L). The ideal salt concentration was determined to be 0.1 g/L, with an optimal stirring speed of 150 rpm. The Taguchi design effectively optimized EC performance, recommending Fe-Fe electrodes for superior efficiency compared to Al-Al and Fe-Al setups.