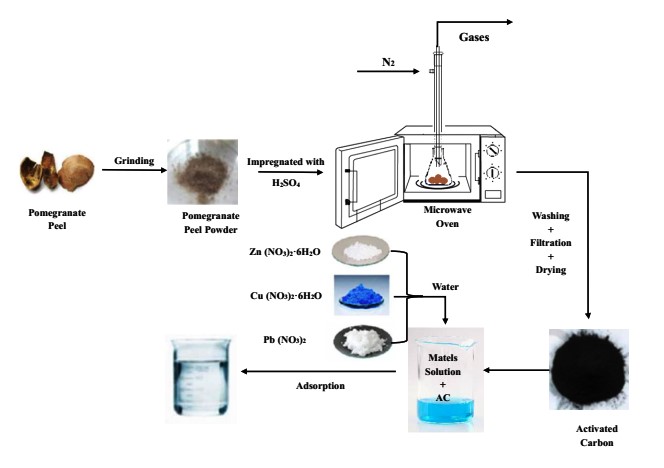
This article presents the simultaneous adsorption of numerous metal ions pb2+, Cu2+, and Zn2+ from synthetic solution by activated carbon produced from pomegranate peel using acid and microwave activation. The effect of absorption time, pH, metal ions concentration, and activated carbon dose on the capacity and adsorption efficiency were investigated. The experimental data were analyzed by Design-Expert software with I-optimal approach. The statistical analysis for removal efficiencies of Cu2+, pb2+ and Zn2+ indicated that the correlation models produced by the software were significant (P- value less than 0.0001) with efficient adsorption. The maximum values of pb2+, Cu2+, and Zn2+ removal efficiencies estimated by correlation models elaborated yield 99.2373 % for pb2+, 98.88 % for Cu2+, and 97.89% for Zn2+ at optimum conditions of adsorption time = 3 hr, pH = 6, metal ions concentration = 30 mg/L, adsorbent dose = 0.3 g/100ml. The adsorption data at equilibrium conditions were fitted with two isotherm models; Langmuir and Freundlich as well as two kinetic models of pseudo first and second order. The results showed good agreement with Langmuir and second order kinetic model.
Total file downloads: 9