- Cite article
- Download PDF
- Share article
- 19 Downloads
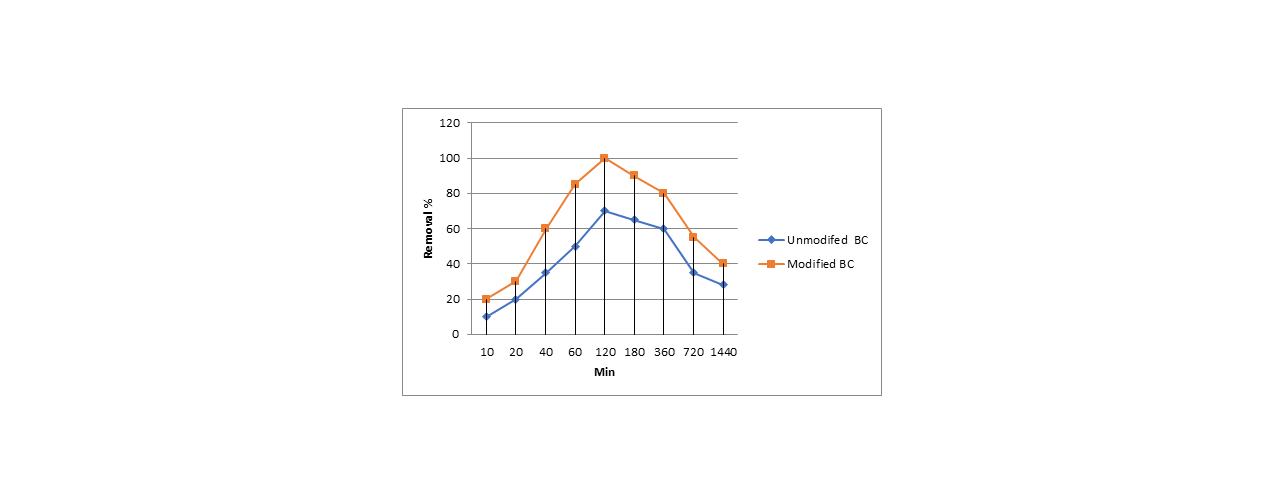
The knowledge of the adsorption-desorption behavior of doxycycline on pyrophosphoric acid modified biochar derived from sesame is limited. In this study, we examined the doxycycline sorption on pyrophosphoric acid modified-sesame stalk biochar. The isotherm and kinetic sorption data showed pyrophosphoric acid treatment enhanced the sorption of doxycycline on both modified and un-modified biochar; and showed the chemisorption including EDA interaction(π-π electron-donor-acceptor) interaction, pore filling and H-bonding might be the primary mechanism. The maximum adsorption capacities for unmodified biochar were (87.6 mg g−1), and 153.9mg g−1 for modified biochar (153.9mg g−1), it was many times higher than pristine biochar. More than 90% of the adsorption capacity was retained after three successive adsorption-desorption cycles. Besides, the strong electrostatic attraction between biochars and doxycycline might largely explain the improved sorption capacity of doxycycline along with increasing pH from 5 to 9. The main responsible mechanisms for the sorption of doxycycline included surface complexation, H-bonding, π-π electron-donor-acceptor (EDA) interactions, pore-filling effects. The results of the current study display that pyrophosphoric acid-modified biochar has potential applications as an efficient, recyclable adsorbent for the removal of antibiotics from wastewater for low-cost remediation.