- Cite article
- Download PDF
- Share article
- 20 Downloads
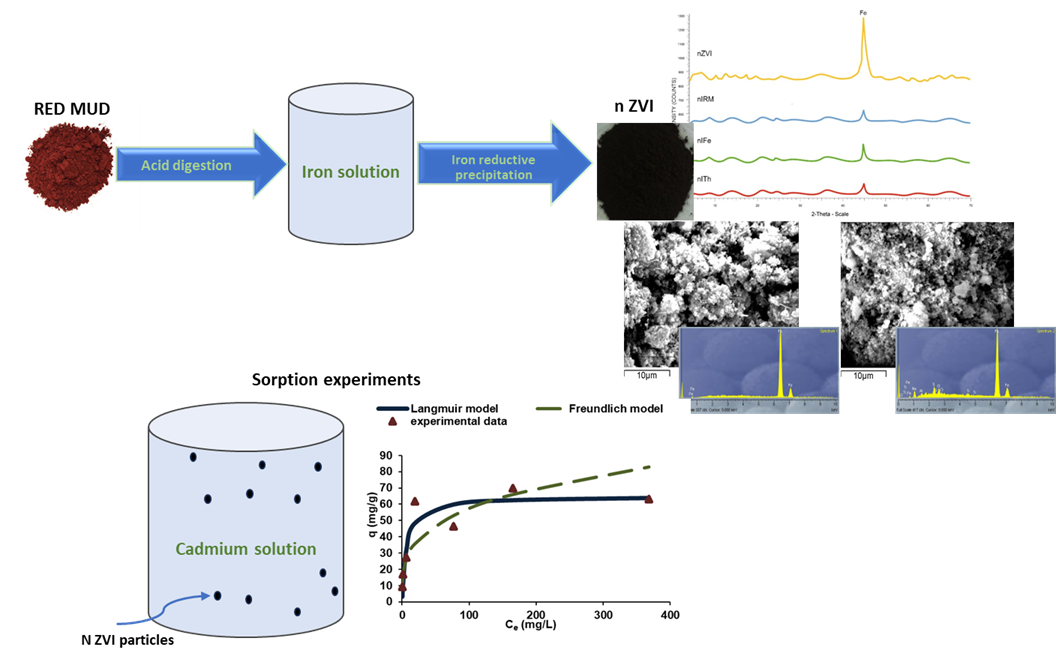
The aim of the present study was to synthesize a sorbent material, nanoscale zero valent iron (n ZVI), using red mud as the iron source in the framework of circular economy. The sorbent synthesized was tested for Cd removal from spiked aqueous solutions. For the synthesis of the sorbent, iron reductive precipitation was used. The specific surface area (BET method) of the sorbent prepared was determined, while its surface composition, morphology, and texture were examined by powder X-ray Diffraction (XRD) and Scanning Electron Microscopy (SEM-EDX). The sorption efficiency of the sorbent produced was inve-1stigated with kinetic and equilibrium experiments, performed in batch conditions. MATLAB software was used to fit experimental data to Langmuir and Freundlich equations. The results of the study showed that with the described process, nanoscale zero valent iron was prepared with grain size 81 and 89 nm and specific surface area of 36.59 and 28.9 m2·g-1 depended on the synthesis procedure followed. The sorbent appeared to be efficient for Cd removal from spiked aqueous solutions. Kinetic experiments proved that equilibrium was achieved within 24h while the maximum sorption capacity as evidenced by equilibrium experiments was 65 mg·g-1. Data proved to follow the pseudo second order model and to fit better to the Langmuir equation.