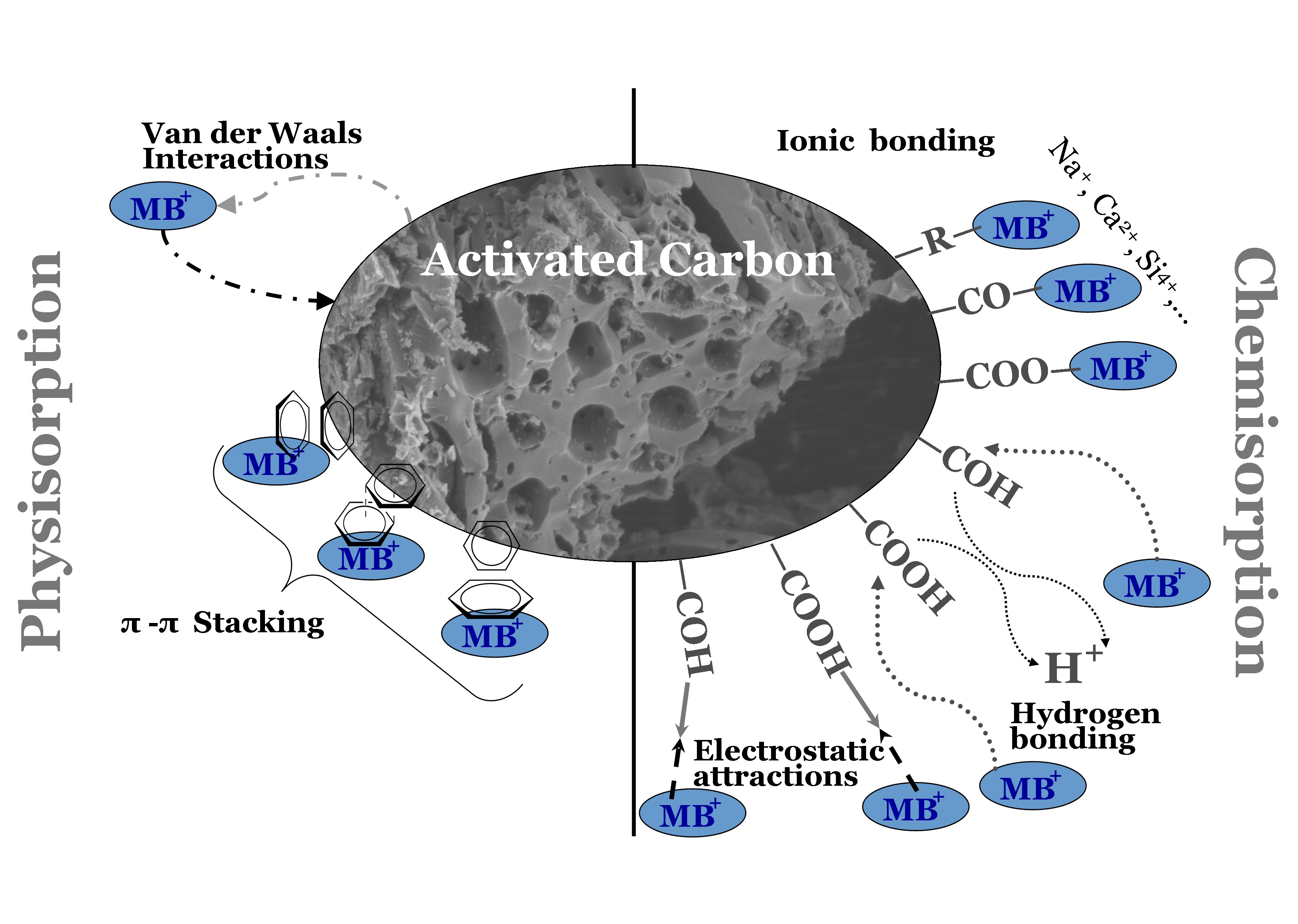
A mesoporous activated carbon was produced from the Azolla Pinnate (AP) seaweed by two-step chemical activation technique using sulphuric acid as activating agent. The adsorption of Acid Brown (AB) from aqueous solutions is examined using the produced carbon (AP). The produced activated carbon renders a homogeneous porous structure, predominantly mesoporous with 686.5 m2/g of BET surface area. The infra-red spectrum revealed AB affinity by multiple functional groups. The point of zero charge and the pH studies evidenced that the surface charge responsible for electronic affinity favours adsorption at higher pH. The SEM and FTIR analysis of AP before and after adsorption of acid brown shows multiple interactions, which is further substantiated by equilibrium, kinetic and thermodynamic models. Equilibrium adsorption data matched best with Langmuir isotherm model, thus primarily follows chemical interaction. However, physical affinity and heterogeneity of surface and species interaction also do exist nearly equally. Pseudo-second order kinetics provided the best explanation of the adsorption kinetics. The temperature variation studies revealed that acid brown adsorption is endothermic with high surface affinity.
Total file downloads: 12