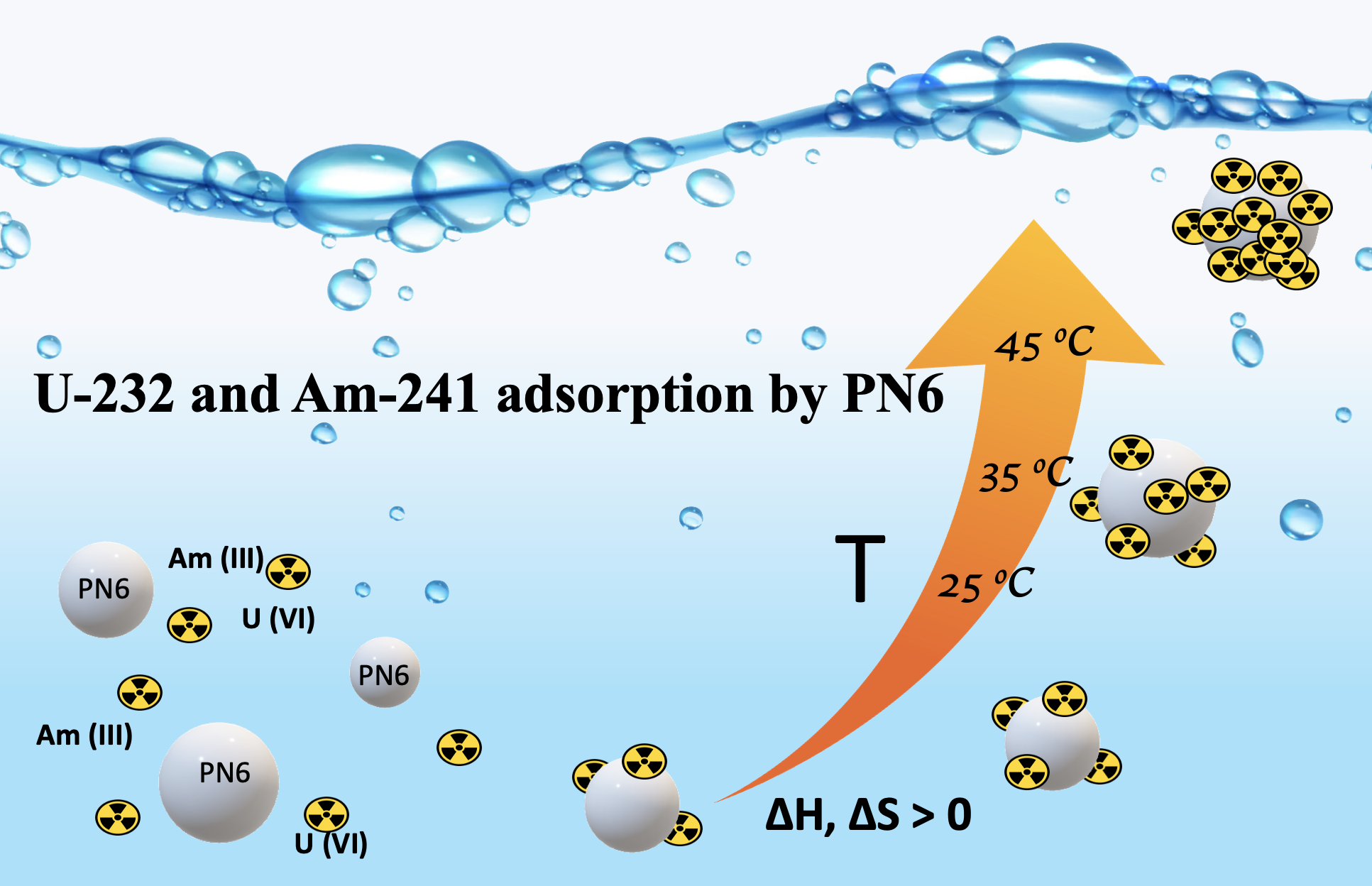
The effect of temperature on the adsorption of U-232 and Am-241 by PN6 has been investigated in laboratory and environmental water samples (e.g. seawater and waste water) in the picomolar concentration range. Generally, increasing temperature favors radionuclide adsorption, indicating that radionuclide binding by PN6 is an endothermic and entropy-driven process. In environmental waters, Kd values are significantly lower than the corresponding values in de-ionized water solutions, because of the presence of various cations (e.g., Ca2+, Fe2+) that compete the radionuclide adsorption by PN6 and the presence of complexing anions (e.g. CO32-), which complex and stabilize the actinide cations in solution.
Total file downloads: 15