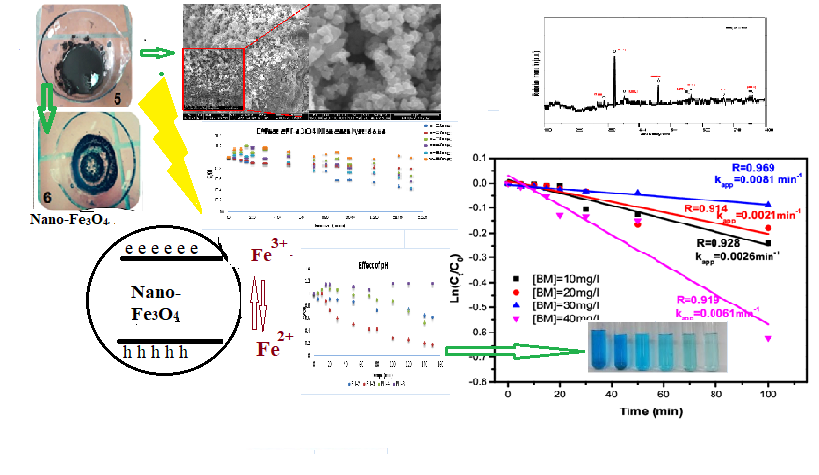
In this research, a simple method for the preparation of Fe3O4 nanoparticles (Fe3O4NPs) with an average size of 38.05 nm via co-precipitation was investigated. X-ray diffraction (DRX), scanning electron microscopy (SEM), and infrared spectroscopy (FT-IR) were used to characterize obtained Fe3O4 nanoparticles. These Fe3O4NPs were then used as nano-catalysts to degrade Methylene Blue (MB) in an aqueous solution via the Photo Fenton-like process. Also, under room solar light and low temperature, the photocatalytic activity of Fe3O4NPs for degrading MB was optimized through various experimental factors such as pH (ranging from 2 to 8), H2O2 concentration (from 10-2 to 5 × 10-1), catalyst amount (20 to 60 mg), and target organic compound concentrations (10 to 40 mg/L). The optimal experimental conditions were found to be a pH of 3, a dye concentration of 40 mg/L, and 40 mg of Fe3O4NPs as nano-catalyst. These conditions led to a high degree of removal (>86%) of MB dye from water. The pseudo-second-order kinetic model was the suitable model to describe the degradation of MB dye with a coefficient value of 0.969. From this, it was concluded that Fe3O4NPs could act as an effective nano-catalyst for a sustainable and environmentally friendly way to eliminate organic pollutants in water and wastewater. Keywords: Fe3O4Nps, structural characterization, BM degradation, photo-Fenton Oxidation process, pseudo-first-order Model, Nanocatalyst.
Total file downloads: 30