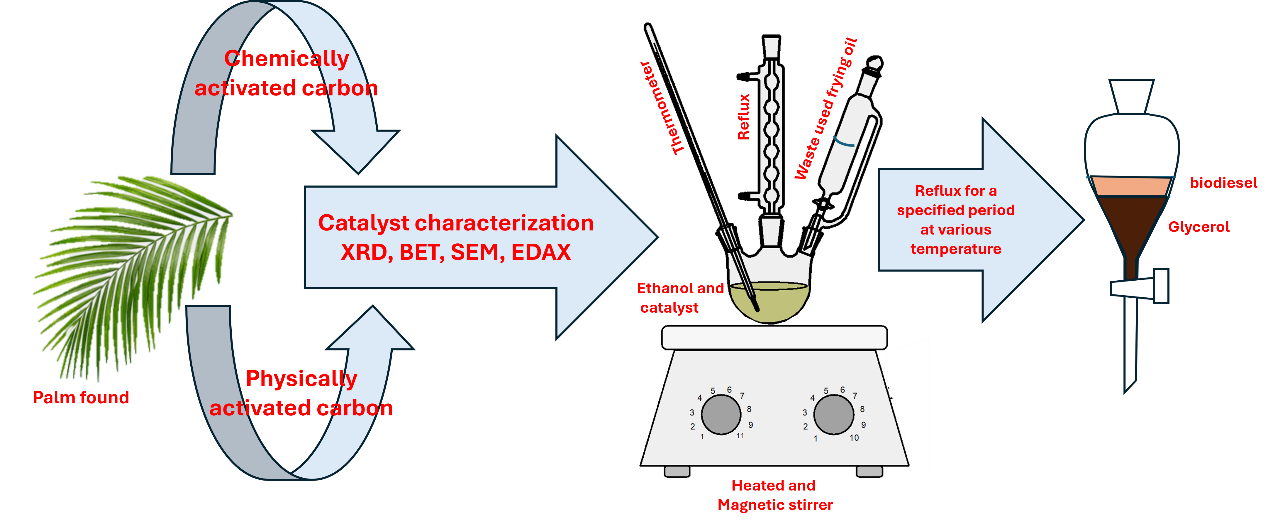
Biodiesel is thought to be more environmentally benign than fuels derived from petroleum, as well as less expensive and able to provide greener energy, all of which have favorably impacted the bioeconomy. Utilizing heterogeneous activated carbon catalysts to produce eco-friendly biodiesel from dried waste date fronds that were prepared and calcined at varying temperatures, waste cooking oil was examined. Brunauer–Emmett–Teller (BET), and thermal gravimetric analysis (TGA) were utilized to characterize this catalyst. The results demonstrated that as the calcination temperature increased, the pore size of the activated carbon catalyst decreased. A transesterification process was employed to optimize the yield of biodiesel (88 wt%). The optimal reaction conditions utilized included a catalyst concentration of 5 wt%, an oil to ethanol molar ratio of 1:10, and a temperature of 80 ◦C for a duration of 3 hours. The validation of fatty acid ethyl ester synthesis was achieved by employing gas chromatography-mass spectroscopy (GC–MS). The potential of fatty acid ethyl ester to function as a suitable substitute fuel is indicated by its compliance with the fuel properties outlined in ASTM D 6751. Consequently, it is commendable to create and execute an environmentally friendly and energy-efficient strategy that employs biodiesel derived from untamed resources and refuse. The adoption and integration of green energy practices could potentially yield positive environmental outcomes, thereby fostering enhanced societal and economic development for the biodiesel sector on a broader scope.
Total file downloads: 19