- Cite article
- Download PDF
- Share article
- 13 Downloads
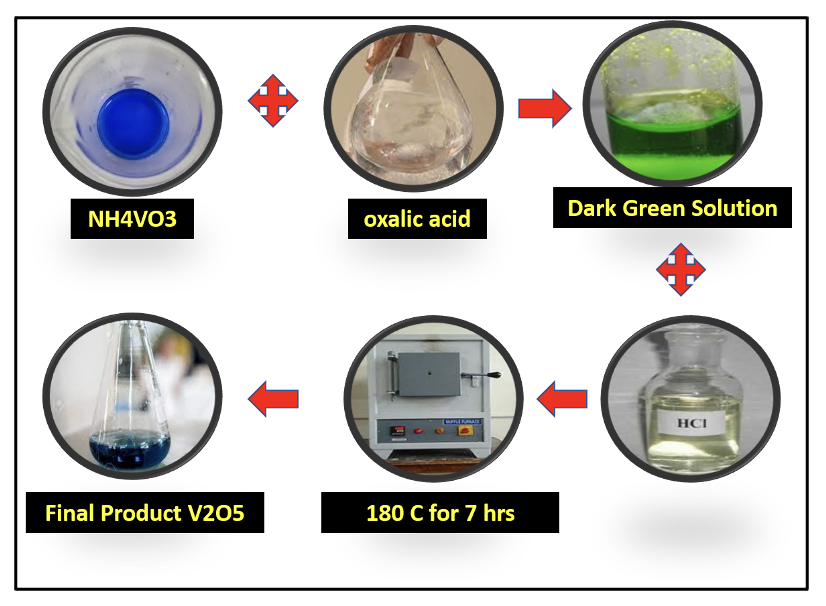
This study employed a simple hydrothermal (HT) approach to synthesize vanadium pentoxide (V2O5) nanomaterials. The inherent limitations of V2O5, including low quantum efficiency and insufficient light sensitivity, restrict its potential for enhanced photocatalytic activity. The study investigates the photodegradation of methyl orange (MO) and Congo red (CR) dyes through annealing. X-ray diffraction (XRD) and Raman spectroscopy confirm the composition of V2O5, while SEM is used to observe the morphology of encapsulated nanoparticles. The bandgap of V2O5 is estimated to be between 2.51 and 2.73 eV using ultraviolet-visible (UV) spectroscopy. Additionally, the photodegradation of methylene blue (MB) dye is analyzed, with calcinated V2O5 achieving a 76% degradation efficiency of MB in 90 minutes. For CR and MO, degradation rates reached 97.91% and 86% within 200 minutes at a 20 mg/L dye concentration. The reaction rate constant for MB degradation was determined to be 8.19 x 10⁻⁵ s⁻¹. Overall, the HT-synthesized V2O5 exhibited enhanced photocatalytic activity due to its improved visible light absorbance, facilitating more effective photodegradation of azo dyes.