- Cite article
- Download PDF
- Share article
- 5 Downloads
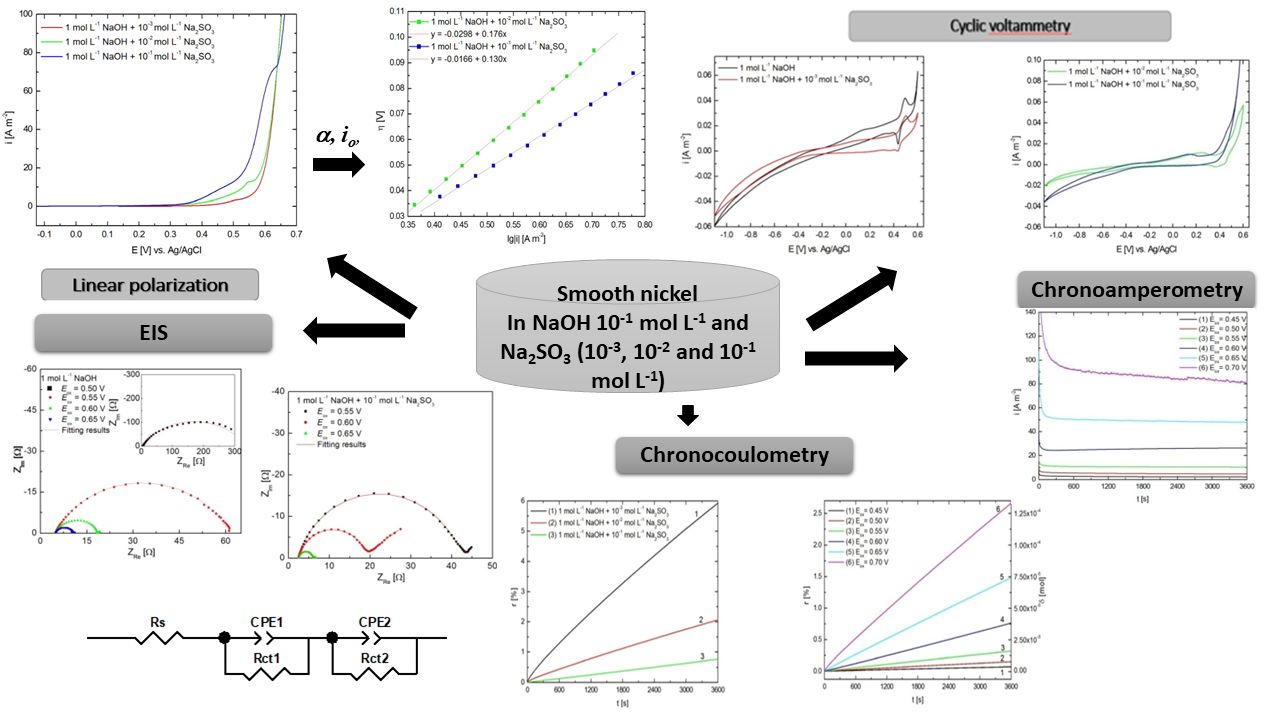
The anodic oxidation of sulphite ions was studied by voltammetric techniques on a nickel electrode in alkaline solution at various concentration of sulphite and polarization rate. The kinetic parameters of the electrode process have been determined using Tafel plots method and a mechanism of the electrochemical oxidation reaction of sulphite has been proposed. Current density, potential range of sulphite electro-oxidation and transformation degree of sulphite ions in the test solutions have been obtained by chronoamperometry and chronocoulometry. The sulphite oxidation mechanism on nickel electrode has been sustained by electrochemical impedance spectroscopy.