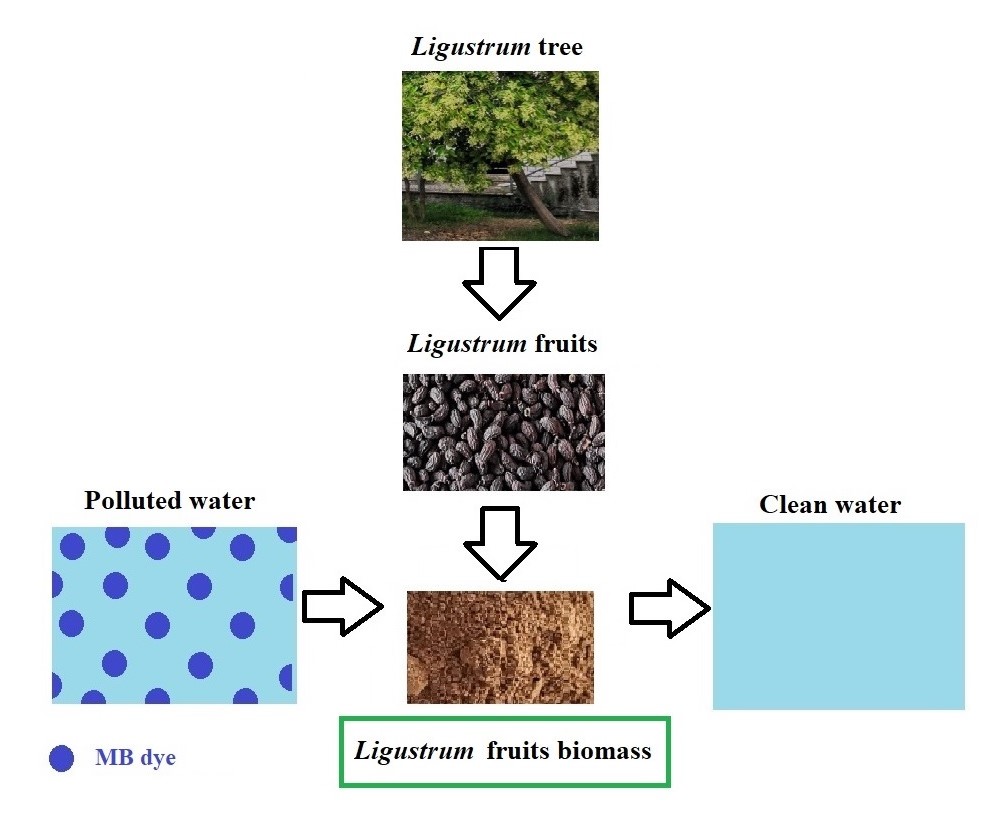
.In this work, biomass from Ligustrum lucidum fruits (LF) was used for the first time as a novel adsorbent to remove the Methylene Blue dye (MB) from aqueous solutions. The adsorption equilibrium was attained after 60 min, and the adsorption followed the pseudo-second-order kinetic model. The rise of temperature from 295 K to 313 K resulted in a decline in the adsorption capacity, suggesting an exothermic process. The Langmuir isotherm model was found to fit better the experimental adsorption data than the Freundlich isotherm model. The maximum monolayer adsorption capacity obtained from the Langmuir isotherm model at pH 5.6 was evaluated to be 95.4 mg/g and 91.8 mg/g at 295 K and 313 K, respectively. The thermodynamic studies indicated that the adsorption was a spontaneous and exothermic process with increasing randomness at the solid/solution interface. Based on FTIR and ionic strength studies, the adsorption of MB by LF mainly occurs via electrostatic interactions and the formation of outer-sphere complexes.
Total file downloads: 10