- Cite article
- Download PDF
- Share article
- 26 Downloads
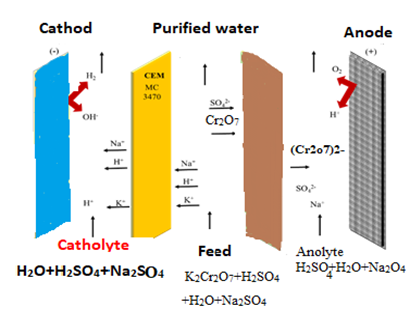
Healthy water is a concern for the world due to rapid population growth and technological advancement. The entry of heavy metals into water as a result of various activities causes water pollution that has persistent effects. In this study, a specially designed electrodialysis cell was used to remove chromium (VI) and nickel ions from wastewater. The chambers were partitioned by Ionac MC 3470 cation exchange and Ionac MA 3475 anion exchange membranes. The cathode and anode were made of carbon fiber and stainless steel, respectively. The effects of voltage, initial pH, time, Na2SO4 concentration, feed flow rate and metal ion concentration on metal removal efficiency, energy consumption, current efficiency, current density and flux were investigated. Optimum values for 98.5% removal of 80 mg/L Cr(VI) ion in 90 min voltage 30 V, pH=3, addition of Na2SO4 0.5 g and feed flow rate 52. 8 ml/min, as observed. At the end of this period, concentration 1 mg/L, energy consumption 40 W/L, flow efficiency 30% and flux 12 x 10.-5 mol/m2s were calculated. Optimum values of 25 V, pH=3, addition of Na2SO4 0.2 g and Qf = 42.6 mL/min were observed for 94.3% removal of 50 mg/L Ni2+ ions in 90 min. At the end of this period, the nickel ion concentration was 4 mg/L, the energy consumption was 34 Wh/L, the flow efficiency was 96.51%, and the flux was calculated as 40×10-5 mol/m2s. This study shows that the electrodialysis method can be effectively used to elimonation chromium (VI) and nickel (II) ions from dilute wastewaters.