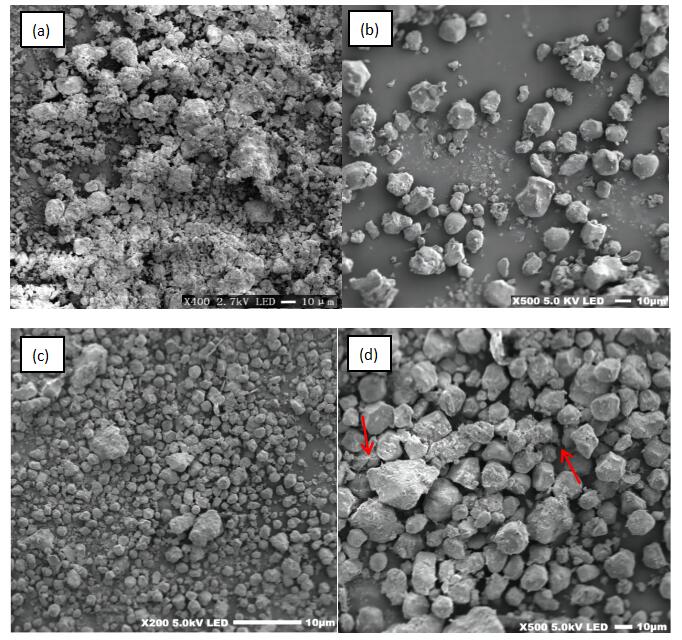
Unqualified treatment of the discharge of wastewater contains a large number of nitrogen and phosphorus and other nutrients, such wastewater into the water environment is easy to lead to eutrophication of water bodies, and cause harm to human health. In order to prepare a cost-effective, efficient and environmentally friendly adsorbent material for nitrogen and phosphorus removal, lanthanum modified montmorillonite(La4-Mt), starch modified montmorillonite(St6-Mt) and starch and lanthanum modified montmorillonite(St6-La4-Mt) were used in this paper to investigate the removal effect and adsorption mechanism of the three modified materials on nitrogen and phosphorus. The mechanism of modification and the properties of the modified montmorillonite were characterised by zeta potential and scanning electron microscopy (SEM) analysis. The test results show that:After the introduction of starch and lanthanum, the adsorption of both nitrogen and phosphorus by the modified montmorillonite was significantly enhanced. The mechanism of PO43- removal by La4-Mt and St6-La4-Mt, materials prepared from La-loaded montmorillonite, is due to the binding of La3+ to OH- in solution to produce La(OH)3, which in turn removes the oxygenate PO43- and NO3- from solution by electrostatic gravitation. The mechanism of removal of oxygenates by St-loaded montmorillonite St6-Mt and St6-La4-Mt is that St itself is an electropositive natural polymeric organic substance, and by reducing the electronegativity of the system, St reduces the electrostatic repulsion between the system and the oxygenates and improves the effect of oxygenate removal, thus achieving the purpose of nitrogen removal and phosphorus removal.
Total file downloads: 11