- Cite article
- Download PDF
- Share article
- 9 Downloads
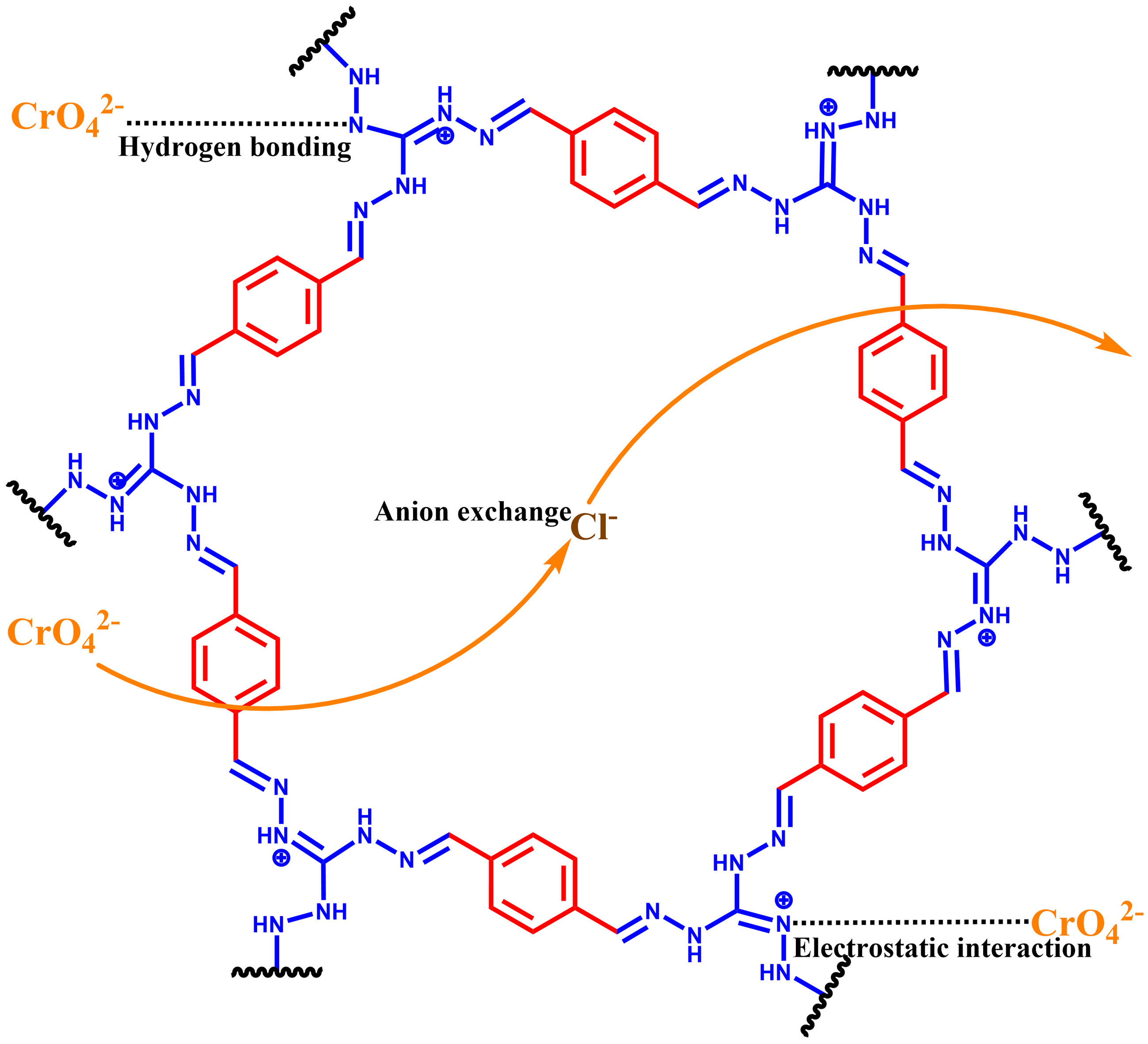
Developing an efficient sorbent for removing the highly toxic Cr(VI) is very important, which tackles the threat of Cr(VI) pollution to ecological environment and human health. Herein, a cationic covalent organic framework bearing cationic guanidine (named TATGCl) was synthesized from terephthalaldehyde (TA) and triaminoguanidinium chloride (TGCl), and exploited to discard Cr(VI) from water. The Cr(VI) removal ability of TATGCl strongly relies on the aqueous pH, and is strong at low pH. The removing ability is obviously also affected by ionic strength. The Cr(VI) sorption process on TATGCl obeys pseudo-second-order and Langmuir models. Batch adsorption experiments and characterization show that the guanidine group on the skeleton of TATGCl is pivotal in capturing Cr(VI) through hydrogen bonding and anion exchange/electrostatic interaction. A reduction mechanism also exists in the Cr(VI) disposal by a small amount of unreacted aldehyde group of TATGCl. The large removal ability makes TATGCl a prospective candidate for discarding Cr(VI) from water.