- Cite article
- Download PDF
- Share article
- 2 Downloads
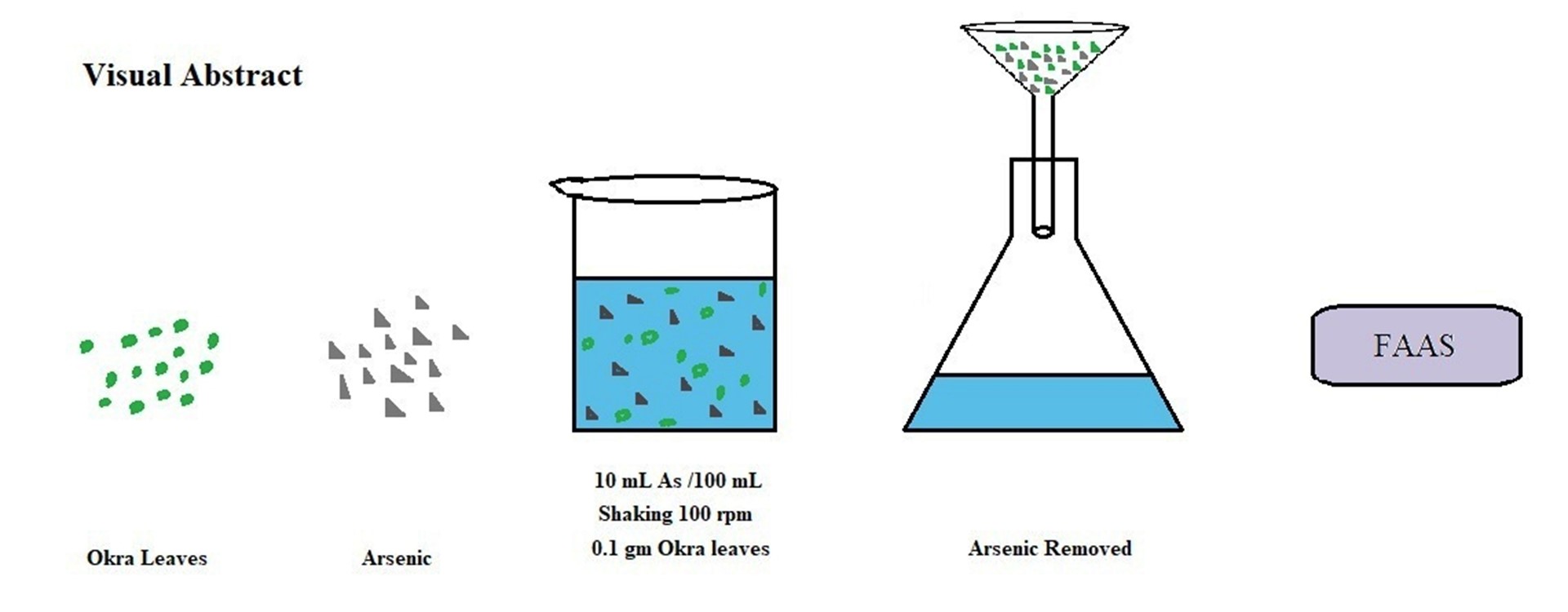
In the present study the sorption efficiency of okra leave sorbent for As(III) and As(V) is demonstrated. Sorption reaction is pH and time dependent. The sorbent shows maximum removal of As(III) and As(V) at pH 7 and pH 6 respectively and equilibrium was achieved at 180 minutes. In isotherm study experimental data were explained by Freundlich and Flory-Huggins models. Maximum sorption capacities calculated by Freundlich Isotherm were 5672.0 μg g-1 and 13160 μg g-1 for As(III) and As(V) respectively. Psuedo-second order rate equation and Morris-Weber equation explained the kinetics of sorption reaction. Due to the presence of heterogeneous active sites on the sorbent, surface sorption as well as intra-particle diffusion occurred. Thermodynamically, sorption reaction was endothermic in nature and proceeded spontaneously. Desorption study revealed that 89.82% of As(III) and 97.11% of As(V) were removed with 1M HCl.