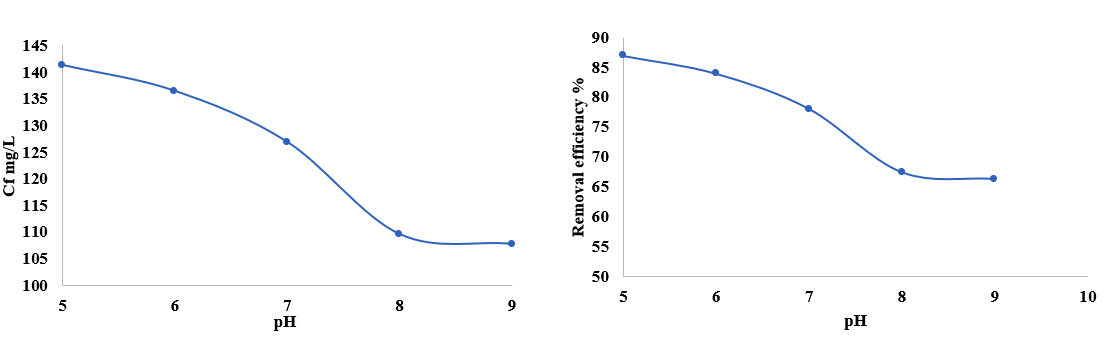
In the present work, the potential of cumnium cyminum as an effective bio adsorbent for the removal of cationic dye (Red 95) was investigated. Batch adsorption experiments were carried out to investigate the effects of adsorbent dosage, pH and contact time with initial dye concentration on adsorption. In connection with adsorbent dosage the highest removal efficiency was 143.64mg/L of removal efficiency 88.36%, regarding pH study the peak removal attained with 141.37mg/L with the removal percentage 86.76% and for contact time study the removal efficiency attained in the concentration of 142.10mg/L in the removal percentage of 87.41%. The adsorption studies demonstrated that the Thomson model, and Yoon nelson model are excellent descriptions of adsorption processes. The maximum adsorption capacity of 143.64 mg/L toward biosynthesized nano adsorbent with removal rates of 88.36% and greatest R2 value of around 0.9879 was well suited to the equilibrium data. The ANOVA was used for the analysis of variance to determine the best parameters for the overall adsorption model. Utilizing SEM, XRD, and FTIR methods, the adsorbent was characterized. Different desorbing agents, such as NaOH, HCl, and NaCl, were examined for the desorption of cationic dye (Red 95) loaded with adsorbent generated from cumnium cyminum, and it was shown that HCl was the most effective desorbing agent when combined with deionized doubly distilled water (DDDW). The proportion removal of cationic dyes was improved with increasing adsorbent dose and contact time and the percent of color exclusion was decreasing with increasing pH.
Total file downloads: 4