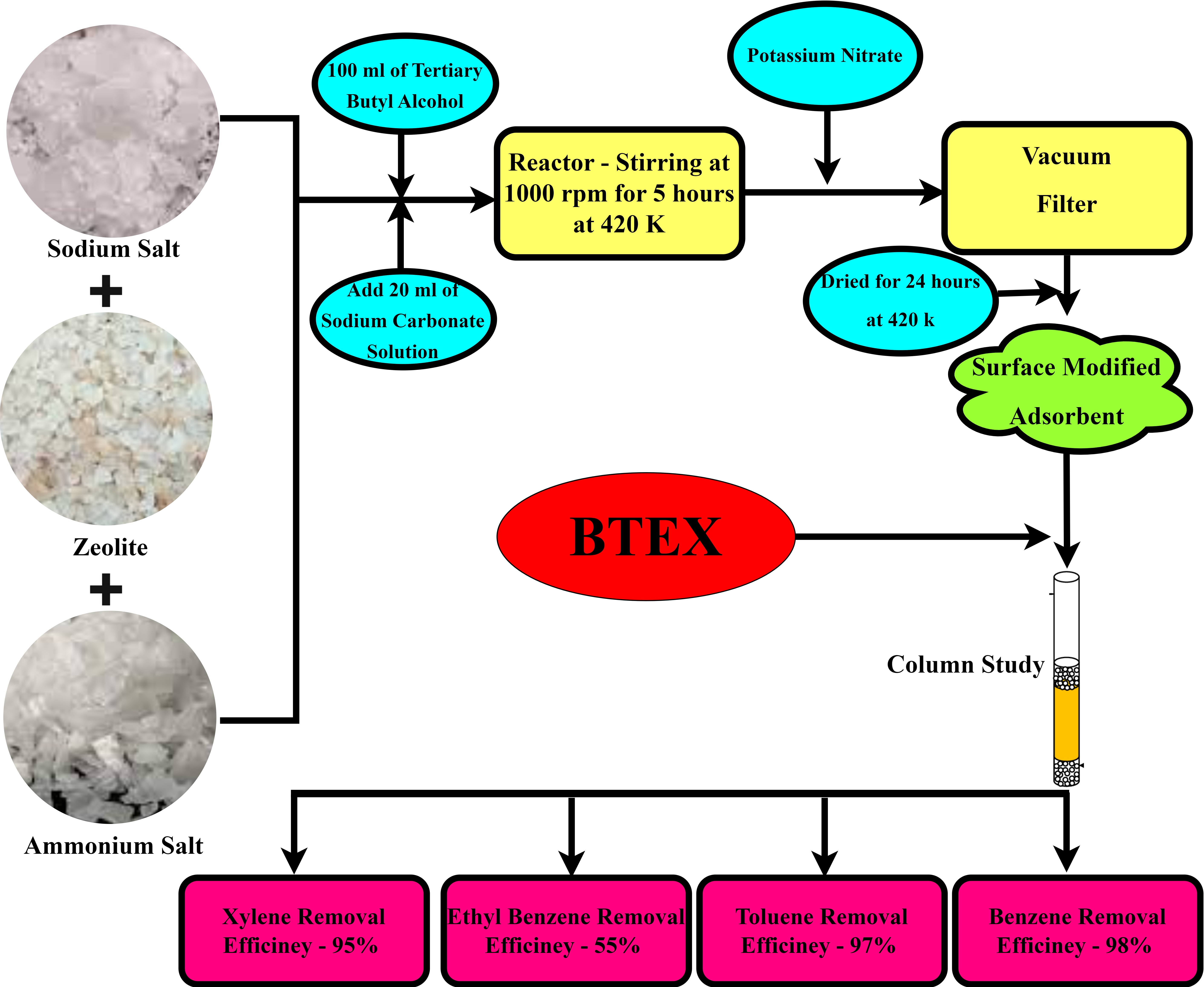
BTEX, also known as benzene, toluene, ethyl benzene, and xylene, is a common gasoline oxygenate. The contamination of groundwater and surface water with BTEX and its primary breakdown product has drawn a lot of attention. However, surface-modified zeolite's sorption mechanisms and affinity for BTEX can be employed to potentially remove these toxins from water. Tert-butyl alcohol (TBA) was utilized to alter the morphology of the adsorbent. Based on the findings, it can be concluded that zeolites have a greater capability for removing emerging chemicals. Some of the emerging compounds' relatively large dimensions limit their natural ability to lower values. Gas chromatography was used to determine the BTEX concentration decrease range. The Yoon-Nelson and Adams-Bohart model's use of mathematical modelling to determine the efficacy of the column's adsorption. The model with the highest R2 values for characterizing equilibrium isotherm data describes equilibrium adsorption data. To gain an understanding of the shape, pore structure, and active sites, the characterisation is concentrated on quantitative analysis using XRD, qualitative analysis using FTIR, and optical study using SEM.
Total file downloads: 14