- Cite article
- Download PDF
- Share article
- 3 Downloads
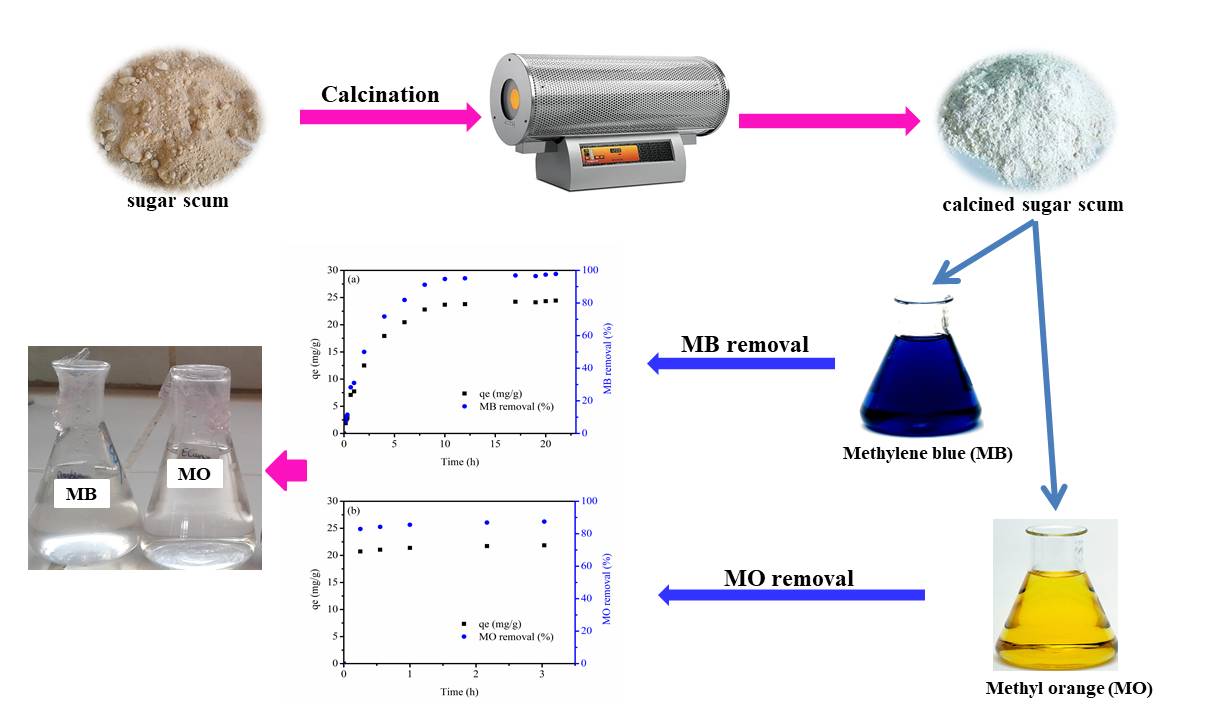
Batch adsorption experiments have been conducted to investigate the adsorption properties of the calcined sugar scum powder for methylene blue (MB) and methyl orange (MO) as models for cationic and anionic dyes. The kinetic and equilibrium adsorption have been investigated to determine the adsorption mechanisms and the adsorption capacities. The kinetic of adsorption was examined by pseudo-first order, pseudo-second order and intraparticle diffusion models. Adsorption isotherm was modeled using Langmuir, Freundlich. The methylene blue adsorption process can be best described by the pseudo-first order kinetic and Langmuir adsorption isotherm models while the methyl orange adsorption process was best fitted to the Langmuir isotherm with a monolayer maximum adsorption capacity of 40.28 mg/g. Moreover, both pseudo-first order and pseudo-second order models could be used to describe the adsorption process of methyl orange. The percentage removal of the calcined sugar scum powder was found greater than 98 % and 87 % for MB and MO, respectively. The results demonstrated that sugar scum is a suitable precursor for the preparation of efficient adsorbent for removal of cationic and anionic dyes.