- Cite article
- Download PDF
- Share article
- 31 Downloads
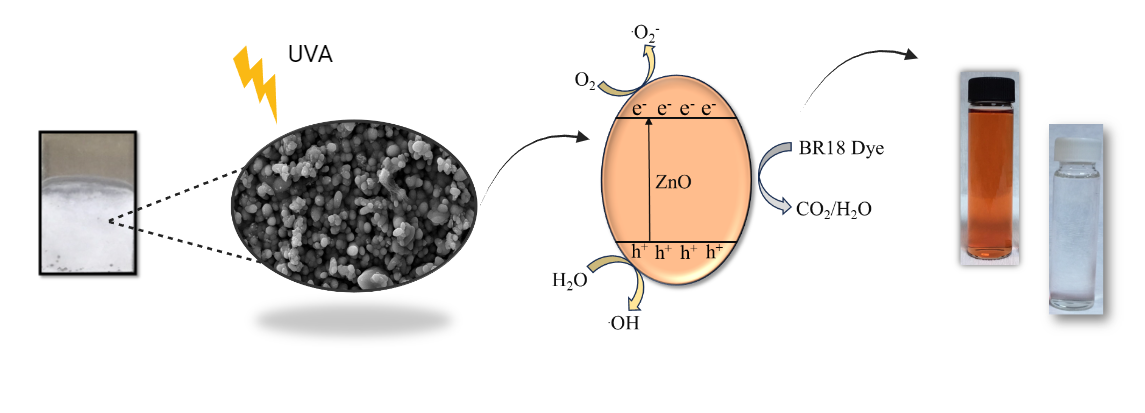
In this study, zinc oxide (ZnO) films have been electrochemically deposited on stainless steel and its photocatalytic activity on the degradation of BR 18 dyes was tested. The effects of different electrodeposition conditions such as deposition potential and deposition time on the nanostructures of ZnO films were investigated in detail and the best electrodeposition conditions were optimized. The electrochemical, structural and morphological, were characterized by cyclic voltammetry (CV), chronoamperometry, X-ray diffraction (XRD), scanning electron microscope (SEM), respectively. The best removal efficiency was 37% at -1.2 V, and increased up to 53% after 300 sec coating. The number of electrodes (1, 2, 3 and 4 coatings) and dye concentration (5, 10, 15 mg/L) covered with -1.2 V potential and the coating synthesized in 300 seconds were studied. A complete removal was obtained when the number of electrodes covered was 4 and at 5 mg/L initial dye concentration. The number of reuses was tested up to 5 cycles. ZnO coatings on stainless steel that were electrochemically charged showed good photocatalytic stability. Because ZnO films have both economic and environmental advantages, it is imperative that they can be synthesized using environmentally benign processes and used in photocatalysis. This novel strategy provides a chance to utilize ZnO film photocatalytic characteristics for a range of environmental remediation applications in addition to providing a sustainable wastewater management solution.