- Cite article
- Download PDF
- Share article
- 5 Downloads
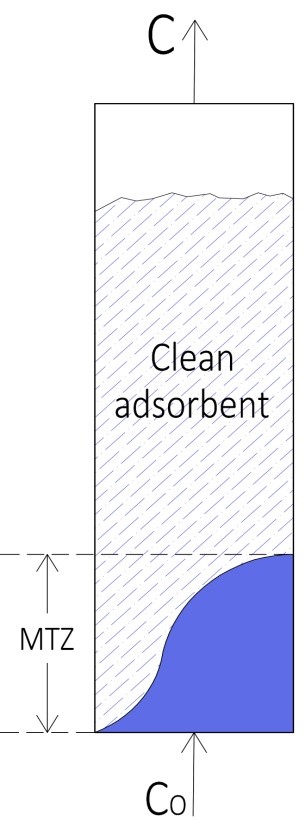
The temperature, feed rate, length of mass transfer zone, utilization factor and partial pressure are the parameters considered for fixed bed sorption of CO2 from N2/CO2 mixture. The breakthrough time relies strongly on the temperature and feed rate. The prolonged breakthrough and saturation times have been realized for AC. The response curves of AC are vastly steep signifying the maximal utilization of bed capacity at the breakpoint. In general, the length of MTZ increases with raised temperature and feed flow rate. The capacity utilization factor reduces with raised temperature and feed flow rate. A utilization factor of 0.919 was determined for AC. The maximal capacity for CO2 reduces significantly with an increased temperature. The maximal capacities of 32.99 gm CO2/Kg was determined at a temperature of 298 K for AC. The capacity improves considerably with CO2 partial pressure and AC exhibited higher adsorption capacity compared to SG. The capacity improves considerably with increased feed rates and maximal capacity of 39.14 g CO2/Kg adsorbent was determined for AC at the feed rate of 8.33 x10-3 m3/sec. Owing to higher sorption capacity and utilization factor, the AC may be used for economical separation of CO2 from N2/CO2 mixture