-
VolumeVolume 22 (2020)
-
Issue
-
Pages369-380
- gnest_02841_published.pdf
-
Paper IDgnest_02841
-
Paper statusPublished
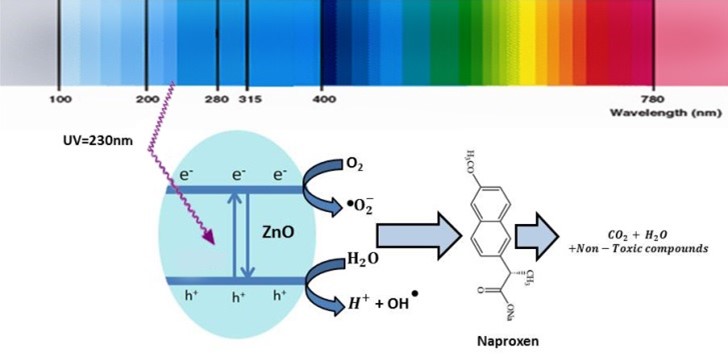
The aim of this study was to optimize the removal of Naproxen (NPX) by the UV/ZnO photocatalytic process using response surface methodology based on Central Composite Design (CCD). The effect of parameters such as ZnO concentration, contact time, pH, temperature, and initial NPX concentration were studied. The ANOVA results indicated high coefficient values of adjusted R2 (0.9843) and predicted R2 (0.9695). The quadratic model with the highest R-squared designation was chosen to predict the NPX removal efficiency of the UV/ZnO process. Under optimal conditions that include an optimum initial NPX concentration of 21.59 mg/L, ZnO concentration of 371.15 mg/L, contact time of 73.92 min, pH of 6.87, and temperature of 24.35°C, a NPX removal efficiency value of 71.19% was obtained. The results show that the removal of NPX is most affected by the variables- initial NPX concentration, time, pH, and ZnO concentration, respectively, but temperature as a variable does not have a significant effect on the efficiency of the process. Moreover, the NPX photodegradation kinetics can be explained through the pseudo-first-order model. The UV/ZnO photocatalytic method has high potential for the removal of NPX, and that CCD is an appropriate method to optimize the operating conditions for NPX photodegradation.
Total file downloads: 3