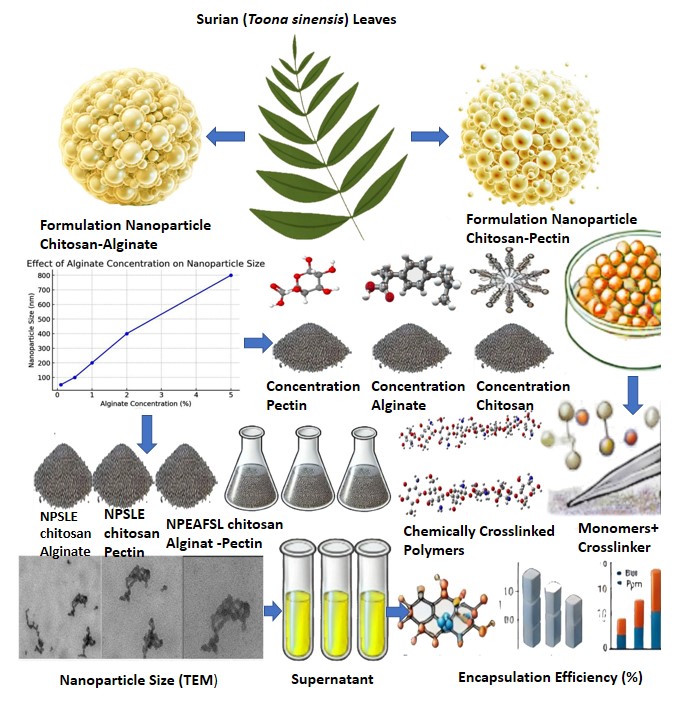
Chitosan-alginate and chitosan-pectin nanoparticles show great promise as delivery systems in the rapidly evolving field of cosmetics. The formulation of these nanoparticles represents a targeted delivery system with the ability to enhance pharmacological activity and improve stability. To optimize the efficiency of natural polymer utilization, we implemented a development approach for chitosan-alginate and chitosan-pectin nanoparticles, aiming to enhance their activity and stability compared to their extract forms. Through ionic gelation, nanoparticles from Surian leaf extract (SLE) and ethyl acetate fraction of Surian leaf (EAFSL) were formed with particle sizes ranging from 172 nm to 200 nm, polydispersity index (PI) ranging from 1.3 to 1.9, and zeta potential ranging from -11 mV to -27 mV. The use of pectin polymer affects the activity of SLE and EAFSL nanoparticles in inhibiting elastase enzymes. This is because pectin envelops or forms a membrane layer on the surface of nanoparticles, causing SLE and EAFSL to be trapped within the nanoparticles and difficult to release for elastase enzyme inhibition. This results in instability during storage at room temperature, tending to precipitate more quickly. In inhibition tests against the elastase enzyme, it was found that chitosan-alginate and chitosan-pectin polymers exhibited the highest % inhibition compared to SLE and EAFSL alone. For instance, SLE nanoparticles with 0.75% chitosan and 1.25% alginate polymers showed a % inhibition of 39.40% against elastase enzyme, whereas SLE alone exhibited only 30.18% inhibition. Similarly, EAFSL nanoparticles with 0.75% chitosan and 1.25% alginate polymers demonstrated an 87.30% inhibition against elastase enzyme, compared to EAFSL alone with only 22.42% inhibition. Additionally, the characterization of SLE and EAFSL nanoparticles with chitosan-alginate and chitosan-pectin polymers resulted in significant polydispersity. As a consequence, the generated zeta potential values were very low, increasing the likelihood of aggregate formation. This occurrence is attributed to the fact that the nanoparticles are still in their nanoparticle form. It is anticipated that in the nano-hydrogel formulation, the zeta potential will be stabilized within the nano-hydrogel structure. This is because the inherent properties of hydrogels enable them to encapsulate the formed nanoparticles into their structure.
Total file downloads: 39