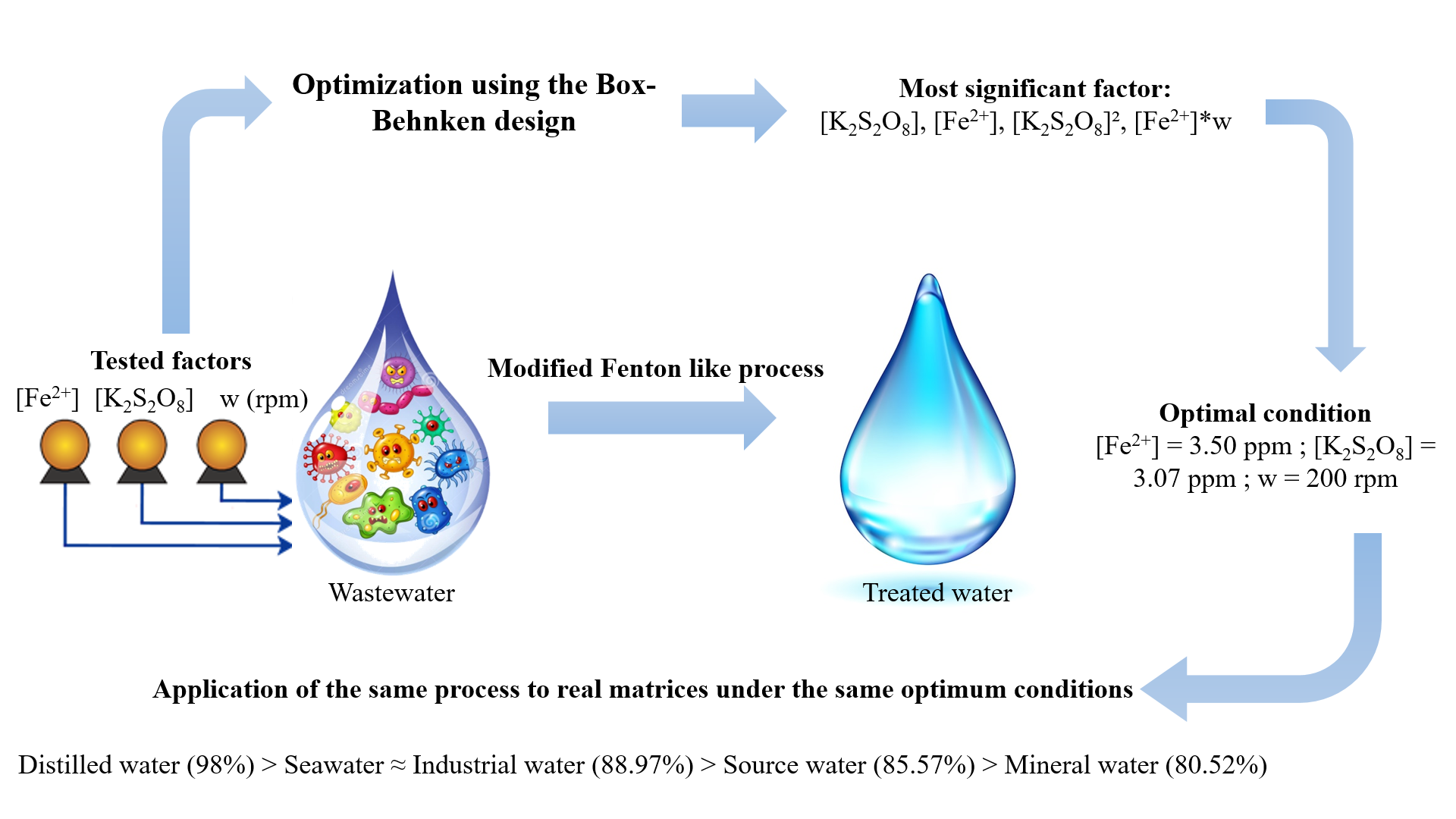
The aim of this work was to investigate an advanced oxidation process for removing malachite green from aqueous solutions using a modified Fenton-like process. An experimental Box-Behnken design was applied to determine the optimal conditions by examining the effects of catalyst concentration ([Fe2+]), oxidant concentration ([K2S2O8]), and stirring speed. The analysis of variance (ANOVA) indicated that oxidant concentration was the most significant factor, with a p-value of 0.001, while catalyst concentration, the quadratic term of the oxidant, and the interaction between catalyst concentration and stirring speed were also significant. The optimal conditions for maximum dye removal were found to be a catalyst concentration of 3.5 ppm, an oxidant concentration of 3.07 ppm, and a stirring speed of 200 rpm, achieving a theoretical degradation yield of 100% and an experimental yield of 98%. This agreement validates the model and the importance of the optimized parameters. Additionally, degradation kinetics studies in various natural waters revealed that oxidation efficiency followed this order: Distilled water (98%) > Seawater ≈ Industrial water (88.97%) > Source water (85.57%) > Mineral water (80.52%).
Total file downloads: 19