You are here
Adsorption isotherm and kinetic studies for the decolorization of sunset yellow FCF dye using economically feasible low cost adsorbent
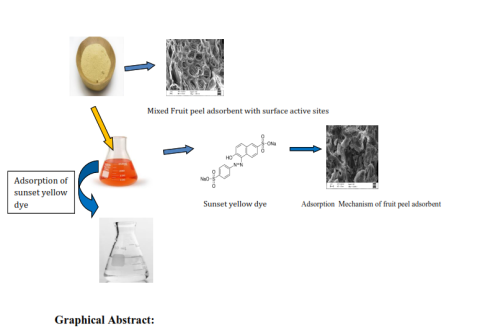
The present communication attempts to explore the adsorption potential of Mixed Fruit Peel Waste (MFPW) to remove Sunset Yellow FCF dye from an aqueous solution. The MFPW is a low-cost adsorbent prepared from the peels orange, watermelon and banana. The characterization of MFPW was made through FTIR, SEM and BET studies. The FTIR studies revealed the presence of functional groups such as nitro, carboxyl, ester, ether, phenol and alkyne that are solely responsible for adsorption. The surface morphology exposed the clear and well-developed pores of MFPW. Batch adsorption studies resulted in a maximum adsorption capacity (qmax) of 200 mg/g at optimum pH 3.0, contact time of 100 minutes, and adsorbent dose of 2.0 g/L with an initial dye concentration of 40 ppm. Sunset Yellow FCF dye removal was discovered to be spontaneous and endothermic in nature, with the Langmuir isotherm and pseudo-second-order-kinetics providing the best fit. In summary, mixed fruit peel waste adsorbent can be used as a low-cost, environmentally friendly and sustainable adsorbent to decolorize sunset yellow FCF dye.
