You are here
Vigna stipulacea mediated Fe nanoparticles synthesis: A greener approach for sequestration of Pb2+ from aqueous environment
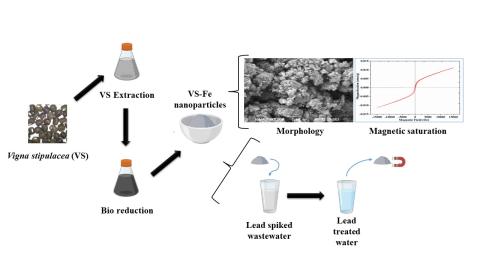
The greener approach offers a viable, sustainable and eco-friendly way to synthesize nanoparticles. This study used the seed extract of Vigna stipulacea (VS) as a bioreducing agent to synthesize iron nanoparticles (VS-Fe). The VS seed extract contains polyphenols and lignin content that acted as a bioreducing agent during VS-Fe formation. The Vigna stipulacea-mediated Fe nanoparticles were characterized using UV, XRD, FTIR, EDAX and BET surface analysis. The as-synthesized VS-Fe, comprised of Fe0 phase and Fe hydroxides, had an average crystallite size of 30.65 nm. It possessed a surface area of 199.189 m2/g and magnetic saturation of 11.21 m emu. The VS-Fe exhibited excellent adsorptive behavior during the sequestration of Pb2+ ions from an aqueous environment. The Pb2+ uptake was maximum (96.7%) under the optimal conditions of 60 min contact time, 0.01 g/ 100 mL VS-Fe dosage and pH 6. The equilibrium data of Pb2+ adsorption was more appropriate with pseudo-second-order kinetics (R2 = 0.9903) and Langmuir isotherm (R2 = 0.9941) with qmax of 1020.50 mg/g. Thus, the dominance of chemisorption in Pb2+ removal was revealed. It was further confirmed with the SEM micrograph of Pb-loaded VS-Fe nanoparticles. Overall, this study demonstrated the inexpensive and non-toxic way of synthesizing Fe nanoparticles and their utilization in effectively removing Pb2+ ions from water.
