You are here
Enhanced photocatalytic degradation of Remazol Black under visible light illumination through S doped TiO2 (S-TiO2) nanoparticles: Operational factors and kinetic study
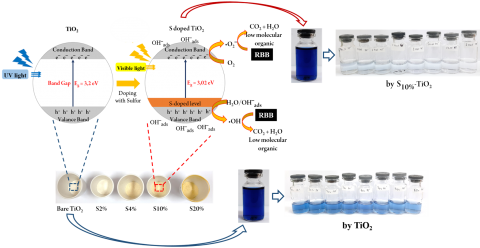
The degradation of Remazol Black (RBB) by S-TiO2 photocatalyst was investigated. X-ray diffraction, fourier-transform infrared spectroscopy, scanning electron microscopy, transmission electron microscopy, and UV-vis specular reflectance spectroscopy has been used to characterize S-TiO2. The results suggested that the optical absorption edge of TiO2 was red-shifted by the addition of S dopants and the bandgap energy was 3.02 eV. The sulfur species were found to be evenly dispersed on the TiO2 crystal lattice as cationic sulfur (S6+) which corresponds to the cationic substitution on TiO2. The particle size decreased to 4-14 nm after S doping, which indicates that the addition of S dopants has contributed to an improvement in the photocatalyst surface area. The degradation of RBB was achieved 94% after 120 min visible light irradiation, a remarkable increase compared to bare TiO2 which was only able to degrade 48% of RBB at the same time. Optimization of the pH showed that the optimum pH for RBB degradation was 3.0, and the photocatalyst dose was 0.8 g L-1. Kinetic study showed that S-TiO2 photocatalytic degradation of RBB followed the pseudo-second-order kinetics model. Reducing the bandgap has been found to increase the activity of photodegradation in the visible light region.
